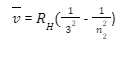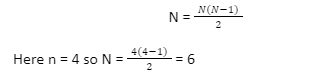As you may be aware that electrons in an atom or a molecule consume energy, get excited, jump to a higher energy level from a lower energy level, and then emit radiation when they return to their initial states. This phenomenon accounts for the emission spectrum through hydrogen as well, known as the hydrogen emission spectrum.
In the late 1800s, it was known that when a gas is excited using an electric discharge and the light emitted is viewed through a diffraction grating; the spectrum observed consists not of a continuous band of light, but of individual lines with well-defined wavelengths. Experiments have shown that the wavelengths of the lines were characteristic of the chemical element emitting the light. They were an atomic fingerprint that resulted from the internal structure of the atom.
Body
The hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. The hydrogen atoms of the molecule dissociate as soon as an electric discharge is passed through a gaseous hydrogen molecule. It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms. The hydrogen emission spectrum comprises radiation of discrete frequencies. These series of radiation are named after the scientists who discovered them.
Hydrogen spectrum wavelength
As we studied earlier, when any hydrogen atom absorbs a photon, it causes the electron to experience some transition to a higher energy level, for example, n = 1, n = 2. When a photon is gained by a hydrogen atom, the electron undergoes a transition from a higher energy level to a lower one, for example, n = 3, n = 2. During the transition from a higher energy level to a lower energy level, the transmission of light occurs. The spectrum is caused by the quantized energy levels of the atoms, to comprise wavelengths that reflect the differences in these energy levels. For example, the line at 486 nm corresponds to the transition, n = 4 n = 2.
Wave number v can be find out by following formula
![]()
Here RH is Rydberg constant
n1 and n2 are principal quantum numbers of two energy levels.
Lyman series:
This series was discovered by Theodore Lyman. This series is a hydrogen spectral series of transitions, resulting in the wavelengths in the ultraviolet region and emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1, where n represents the quantum number. The transitions are named on the basis of Greek letters:
Lyman-alpha is called when transition takes from n = 2 to n = 1
Lyman-beta, n = 3 to n = 1
Lyman-gamma, n = 4 to n = 1
Balmer series
The Balmer series is a series of spectral emission lines of hydrogen that result from electron transitions from higher energy levels down to the energy level with principal quantum number 2. Out of all the transitions, there are four transitions that are visible in the optical waveband, which are empirically given by the Balmer formula:
![]() Paschen series
Paschen series
When an electron jumps from any higher orbit, i.e., if n1= 3 and n2= 4,5,6…, the series obtained is called the Paschen series. Discovered by German physicist Friedrich Paschen in 1908, all the Paschen lines lie in the infrared region. This series of lines overlaps with the lines of the Brackett series, i.e., the shortest line of the Brackett series has a wavelength that is equal to that of the Paschen series. Most of the subsequent series overlap. It is given by:
It lies in the infrared region.
Brackett series
When an electron jumps from any higher orbit to the fourth orbit, i.e., n1= 4 and n2= 5,6,7, the series obtained is called the Brackett series. Named after the American physicist Frederick Sumner Brackett who first observed the spectral lines in 1922, the spectral lines of the Brackett series lie in the far-infrared band. It is given by:
It lies in the infrared series.
Pfund series
When an electron jumps from any higher orbit to the fifth orbit, i.e., n1= 5 and n2= 6,7,8, the series thus obtained is called the Pfund series. Experimentally discovered in 1924 by August Herman Pfund, it is given by:
It lies in the infrared region.
Conclusion
The explanation of the hydrogen spectrum prepared by Niels Bohr was a brilliant achievement for modern quantum theory. It greatly stimulated progress. Bohr was awarded the Nobel Prize in 1992 for excellent work in Physics. Following are the wavelength equations for different series:
For Lyman series =
For Balmer series =
For Paschen series =
For Brackett series =
- For Pfund series =
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





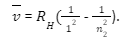
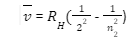 Paschen series
Paschen series