Electrostatics is the branch of physics that deals with the charges that are either not in motion or are very slow-moving. For instance, the charges that attract two balloons are very slow and are an example of electrostatics.
The observations made in the 18th century, such as charges either attract each other or get repealed or the decreasing distance between charges increases, gave rise to Coulomb’s Law of Electrostatics. The law stands to be the first explanation and identification of electrostatics. It stands as the fundamentals for the derivation of other laws, such as Gauss’ Law.
In the below information, there will be the elucidation of what electrostatics is. Further, there will be a complete derivation of Coulomb’s law for electrostatic charges and it’s working for various quantities. Better understanding will be interpreted with practical examples of electrostatic forces.
What exactly is Electrostatics?
Electrostatics is the branch of physics that deals with stationary or slow-moving charges; for instance, when the paper attracts a ruler, the force is weak and shaky. Here, there are electrostatic charges that hold the paper to the ruler. At the same time, the force is weak and can break off easily. The phenomenon that occurred by these charges is described by Coulomb’s Law theoretically.
Coulomb’s Law
Coulomb’s law defines the electrostatic forces in terms of repulsion and attraction. It is a kind of inverse square law, like the gravitational force. Coulomb’s law of electrostatics states that the magnitude at which electrostatic charges repel or attract is “directly proportionate” to the magnitude of charges when multiplied. It is also proportionally inverse to the square distance between the charges.
Let us now get to the derivation of Coulomb’s Law, shall we? The derivation will help in understanding how the law is implemented mathematically.
Let q1 and q2 be the two-point charges. q1 is the source charge, and q2 is the test charge. And r is the distance between the point charges.
F gives the force of attraction/repulsion between the two point charges.
Thus,
F ∝ q1q2
And, F ∝ 1/r2
Therefore, we can say that –
F = k q1q2/ r2
Where k is the constant for proportionality equal to 1/4 π ε0, it presents the vacuum’s ability to store electric energy. The value of k results in 9 × 109 Nm2/ C2. As we consider the S.I. unit for ε0 value, it is calculated as 8.854 × 10-12 C2 N-1 m-2.
Vector form of Coulomb’s Law
Let us consider two charges, q1 and q2, at r1 and r2, to be their position vectors, respectively.
Since they have similar charges, they won’t get attracted to one another.
Let the force of q1 on q2, be F12 and the force of q2 on q1 be F21.
Therefore, the vector from q1 to q2 is r12.
r21 = r2 – r1
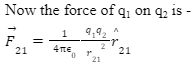
Now that is the vector form of Coulomb’s Law.
What is An Electric Field?
An electric field is defined as the region where the force is applied. Electric fields vary, spending on the area where the force has been applied last. Lines usually help us to visualize what exactly the electric field looks like. It moves from the positive to the negative point of charge. These lines are parallel to the electric field. The lines are the regions that surround the point-charged particles. If we view it from one point, from where the charge is flowing, the lines move radially outwards.
Positively Charged Particles
Here, the number of positively charged ions, i.e. protons, exceeds the number of negatively charged ions which are the electrons. Neutralization of this field occurs when the negative charge equals the positive charge in the field.
Negatively Charged Particles
In this case, negatively charged particles, i.e. electrons exceed the number of positively charged particles which are the protons. More electrons are needed and need to be equal in number to neutralize.
Neutral Particles
Neutral are those particles that do not have any charge. They are called neutrons. However, a neutral field will be formed not just by neutrons, but also by an equal number of electrons and protons.
Example Showing Electrostatics Forces
- Rubbing a rod against the cloth
- Nylon clothes can sometimes get attached to the skin due to extreme rubbing
- Photocopy
- Television screen after it is just turned off
Conclusion
There is a complete elucidation of what electrostatics is in the above information. Electrostatics is the branch of physics that deals with the charges either not in motion or are very slow-moving. For instance, the charges that attract two balloons are very slow, and are an example of electrostatics.
Coulomb’s law of electrostatics states that the magnitude at which electrostatic charges repel or attract is “directly proportionate” to the magnitude of charges when multiplied. It is also proportionally inverse to the square of the distance between the charges.
Coulomb’s law of electrostatics is essential to understanding the relation and proportion between two electrostatic charges. The law is the first study that investigated electrostatic forces.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




