In nature, charge is the physical property of matter which exhibits different characteristics. When we rub our hair over a silk cloth, the crackling sound appears with the exertion of our hair towards the cloth, as if an attraction is caused towards it. This is mainly due to the famous property of multiple electric charges that, like charges attract and unlike charges, repel.
When a number of charges are interacting, the resultant force on a particular charge is given by the vector sum of the forces produced by the individual charges.
If several point charges q1, q2, q3…qn simultaneously exert electric force on the charge q, then the net force on ‘q’ is obtained by taking the vector sum of the individual forces.
What is Coulomb’s Law?
It states that the magnitude of the force between two point charges q1 and q2 and describes the force to be in direct proportionality with the product of the two charges and in inverse proportionality with the distance that separates the two charges. It was discovered in 1785 by Charles-Augustin de Coulomb, a French physicist. It is also called the law of electrostatic force.
Coulomb’s law equation :
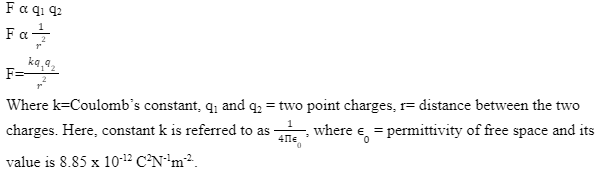
Coulomb’s law exhibits only interaction between two charged particles. So, what will happen if there is a third charge exerting force?
The law of Coulomb forces between multiple charges comes into play here.
The experimentally proven law quantifies that the effect of the exertion of force by two charges simultaneously on a third charge can be calculated by the vector addition of the two forces being exerted on the charge.
This main property is called the Superposition Principle. It holds well for multiple charges. This is the foundation of electrostatics.
Characteristics of Coulomb’s Law

Limitations of Coulomb’s Law
- The charges should be point charges and should be at rest.
- The distance between the charges is supposed to be more than 10-15m (nuclear radius).
- The Coulomb Law of charges is not considered a universal law because it is applicable for short range only..
- Charges should be in an arbitrary shape. If not, it is not possible to calculate the distance between the charges.
Conditions for Stability
When two Coulombic forces are acting on a charged particle, there are certain conditions that need to be met in order to maintain equilibrium. Let us try to understand this with an example. If there are two charges, A and B, placed in space. Let us consider that these two charges are of equal magnitude and are of the same polarity.
The interaction of these two charges will create an electric field where the combined force due to both the charges will be felt due to the superposition principle. Now, if a third unit charge is brought in the field of these charges, what conditions must be met such that the three charges are in equilibrium? There are two movements that the third charge can make, an axial movement towards either of the two source charges and perpendicular displacement between the two charges.
When a third charge is placed, which has opposite nature to charge A and charge B, such that it is at the centre of the two charges, it will experience the two forces Fa and Fb (Fa is force due to charge A and Fb is force due to charge B) as equal. If the third charge is brought closer to either charge, it will be moved from its initial position, and the magnitude of one force will increase, and that of the other will decrease. This will mean that the third charge will not come back to its initial position. Hence, we can say that the axial equilibrium is unstable.
But if the charge were to be displaced perpendicularly, the magnitude of both the forces acting on the charge would change by an equal amount. Therefore, the two charges would bring the third charge back to its original position. Hence, the perpendicular equilibrium is stable.
Conclusion
Coulomb’s Law is the magnitude of the force between two point charges q1 and q2 and describes the force to be in direct proportionality with the product of the two charges and in inverse proportionality with the distance that separates the two charges.
When two Coulombic forces are acting on a charged particle, there are certain conditions that need to be met in order to maintain equilibrium. Conditions for stability are:
(i) The axial equilibrium is unstable.
(ii) The perpendicular equilibrium is stable.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




