Temperature is the measure of the microscopic kinetic energy of atoms and molecules in a body. Heat is described as the energy transferred to a system of interest due to a temperature difference. Heat is essentially energy in transit.
Heat can also be transferred as the diffusion of energy between two bodies at different temperatures that are in direct contact with each other. This is called conduction. Different materials conduct heat differently. The coefficient of thermal conductivity gives the measure of the conduction of heat by different materials.
Conduction
Conduction is the process by which heat is transferred through direct physical contact by the excitation of neighbouring molecules. Molecules and atoms with greater kinetic energy excite the neighbouring molecules and impart them a part of their kinetic energy.
Heat current iH is defined as the amount of heat Q transferred per unit time t in a given material across a temperature difference at a steady state.
iH =dQ/dt
Suppose an object is kept at a temperature difference ΔT and has attained a steady state. Then, for heat conduction, Heat current iH is proportional to the temperature difference ΔT
iH ∝ ΔT
Which implies
ΔT=iH RH
Where, RH is the thermal resistance (which has SI unit K s J-1) to heat flow offered by an object at a temperature difference of ΔT.
Notice that the above equation is mathematically equivalent to Ohm’s law V=IR, where V is the voltage difference across a path that offers a resistance R for the current I.
We observe that Thermal Resistance RH is the property of the object, i.e., dependent on the dimensions of the body, and not a general property of a material. For this reason, we define the coefficient of thermal conductivity.
Thermal Conductivity
The coefficient of thermal conductivity k is related to thermal resistance as follows
k=LRH A
Where, RH is the thermal resistance, L is the length of the body, and A is the cross-section through which heat flow occurs. The heat conduction equation thus becomes,
dQ/dt=kA/L ΔT
Or equivalently
iH =kA/L ΔT
We define the coefficient of thermal conductivity of a material as the amount of heat transferred in 1 second across a slab of area 1 m2 and width 1 m, which results in 1 Kelvin rise in temperature.
The coefficient of thermal conductivity is a general property of a material.
The higher the coefficient of thermal conductivity, the higher is the ability of a material to conduct heat.
The S.I unit of thermal conductivity is J S-1 m-1 K-1.
Thermal conductivities of different materials vary slightly with temperature.
Comparing Materials Based on Thermal Conductivity
The coefficient of thermal conductivity has use in many practical applications.
Materials with high values of thermal conductivities are called thermal conductors and are used in applications where heat transfer is highly desirable. Pots, Pans, cooking utensils, geysers, etc., are all examples where high thermal conductivity is desirable. Metals such as copper, aluminium, silver, and steel are good thermal conductors.
On the other hand, materials that have low values of thermal conductivity are called insulators. Insulators find applications where heat flow is not desirable, such as in winter clothes, building materials, on the handles of kitchen utensils, etc.
The coefficient of thermal conductivity varies from 406 J S-1 m-1 K-1(Silver ) for a very good conductor to 0.016 J S-1 m-1 K-1(Argon) for a very good insulator. Wood has a thermal conductivity of 0.12 J S-1 m-1 K-1, while the coefficient of thermal conductivity of water is 0.8 J S-1 m-1 K-1.
Thermal conductivity of a Compound Bar
Let’s see how the coefficient of conductivity is transformed when two slabs of different materials are joined together.
This is the case where two slabs are arranged one after the other (a series combination), as shown in the figure. The heat flow is indicated by the arrow.
The temperature difference across the two slabs varies, most notably at the junction.
Then,
(Temperature difference)both slabs = (Temperature difference)slab 1 + (Temperature difference)slab 2
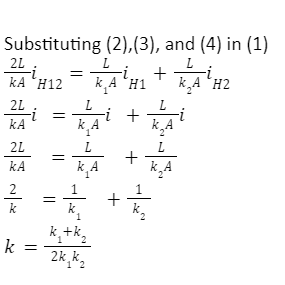
T2 – T1= ( T– T1 ) + (T2 – T) …..(1)
Applying the heat conduction equation individually to the two slabs. Let heat current in slab 1 be iH1 and heat current in slab 2 be iH2
iH1=k1A/L(T-T1) …..(2)
iH2=k2A/L(T2–T)…..(3)
If the net conductivity of the two slabs is represented as k . The heat conduction equation applied to both the slabs would be
iH12=kA/2L(T2–T1)…..(4)
From the law of conservation of energy, the rate of heat energy transferred across both slabs will be equal.
Thus, the net heat current will be
iH12 =iH1=iH2=i
Conclusion
The measure of temperature is the microscopic kinetic energy of molecules and atoms that is able to transfer to neighbouring molecules and atoms in a process known as heat conduction. The rate of heat conduction varies for different objects and materials. We define a heat conduction equation as one that describes the heat flow for a body of material of known dimension and placed under a temperature difference. The coefficient of thermal conductivity is a constant that appears in this equation. Its value describes how easily heat transfer can take place for different materials. Furthermore, we discussed the applications of thermal conductivities in describing thermal conductors and insulators.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




