If we focus on our surroundings, we will find that all matter present in nature is formed by molecules or atoms, which have the property of absorbing energy and emitting it. This property is to move from its lower energy level to higher energy. When these molecules or atoms return to their original state, radiation is emitted from those substances.
This type of nature is also seen in hydrogen elements, mainly due to the emitted spectrum of hydrogen, which also describes the hydrogen energy level. It can mainly be named the hydrogen emission spectrum. The hydrogen atom is claimed to be stable based on the electron quantum number present when the electron is present in the first orbit with the quantum number n=1, which moves around the nucleus.
This state of the hydrogen atom’s orbit is called its ground state, enabling the electron to gain energy from its surroundings. This energy forces the electron to move to the next or higher class, called the excited state. The atom is found to be unstable in the excited state. Similarly, when electrons return to their lower orbits, they show stability, Where the emission of energy is also seen. We denote the value of this energy emission by h, also known as the frequency of radiated energy.
What Exactly Is The Hydrogen Spectrum?
Hydrogen has a significant amount of energy due to hydrogen emission spectrum properties. Like all atoms, the presence of electrons can be seen in the hydrogen atom, which is present in its nucleus. The electric potential between their proton and electron can also be observed, due to which the electron can fulfil quantum values. We use Neil Bohr’s model to help identify quantum fields in chemistry.
It describes how electrons move around and around the nucleus. Changes in quantum mechanics also showed that electrons do not reside in an orbital rather than an orbital path; they have the same energy level as stated in Bohr’s law. The hydrogen emission spectrum describes the amount of energy present in hydrogen, in which electrons move from a higher-energy state to a lower-energy state.
The wavelength of the hydrogen spectrum and hydrogen atoms have different energy quantum levels.
The electron experiences a transition to a higher energy level only when we find that a hydrogen atom can absorb a photon. If we look at n = 1, n = 2, we find that a hydrogen atom emits a photon, where the electron undergoes a one-to-one transition. Hydrogen atoms have different energy quantum levels, where the quantized energy level of the atoms is known, as well as wavelengths in the spectrum that reflect the difference in different energy quantum levels in hydrogen atoms. For example, the line transition at 656 nm is taken where n = 3 corresponds to n = 2, representing the different energy quantum levels in hydrogen atoms. Johannes Rydberg describes the calculation of the wavenumber of hydrogen spectral line emission due to the transition of an electron from one orbit to another.
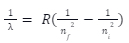
Where,
nf = 1,2,3,…
ni = nf + 1
Rydberg constant for hydrogen has been fixed as 109,677cm-1
The Spectrum of Hydrogen Emissions
Balmer was the first to give a formula for the spectrum based on the wavenumber of the spectral lines emitted in an atom and the energy shells involved, known as the Balmer series. It mainly describes the series of lines in the electromagnetic spectrum related to the visible region of the atom. The basic form of the Balmer series we can also see in the hydrogen emission spectrum is the excitation of an electron from one shell to another. Indicates the situation. Here some chains related to the transition are described as follows;
Series | Transition State |
The Lyman series | Transition from the first shell to other shells |
Balmer series | Transition from the second shell to other shell |
Paschen series | Transition from the third shell to other shell |
Bracket series | Transition from the fourth shell to other shell |
Pfund series | Transition from the fifth shell to other shell |
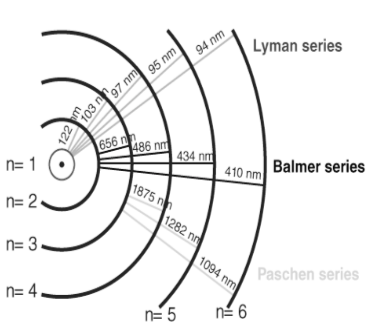
Diagram of the Hydrogen Spectrum
In chemistry, the property of moving an electron to a higher energy level or higher energy orbital of an atom can be seen in any substance, mainly due to the addition of thermal energy or electrical energy, the same thing we see in the hydrogen atom, which We can link to the hydrogen energy level. Here we find that the electrons excited to the steady-state return to the ground state, which can be seen from the emission spectrum of the hydrogen spectrum diagram with a specific frequency of radiation. These things related to hydrogen have been described through diagrams, which mainly describe the hydrogen energy level;
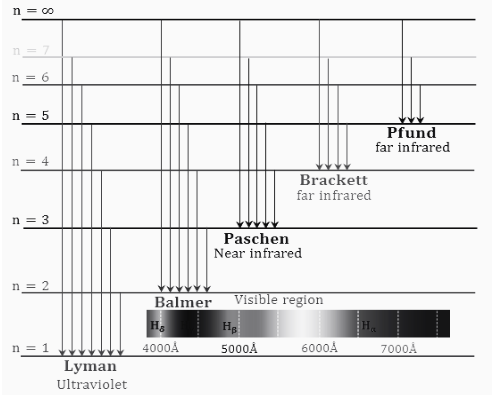
Conclusion
The hydrogen spectrum is a line emission spectrum with a discontinuous number of lines occurring in distinct wavelength regions. The hydrogen spectrum is crucial evidence for demonstrating an atom’s quantized electronic structure. When an electric discharge is conducted across a gaseous hydrogen molecule, the molecules’ hydrogen atoms dissociate. The hydrogen spectrum is crucial evidence for demonstrating an atom’s quantized electronic structure. When an electric discharge is conducted across a gaseous hydrogen molecule, the molecules’ hydrogen atoms dissociate.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




