Dielectrics are materials that are very poor conductors of electric current. These are materials in which the outer electrons are firmly bound to their respective nuclei. These electrons cannot leave the nucleus, even if they can be pulled towards a particular side of the nucleus. They are insulators and contain no free electrons. In stark contrast to insulators are conductors, in which the electrons are free to move from atom to atom. Here, we shall focus on dielectrics and see why they are materials that are very poor conductors of electric current.
Understanding dielectrics and their examples
Dielectrics are non-conducting materials that are very poor conductors of electric current. Moreover, they have a negligible number of charge carriers. Fundamentally, they are insulators and contain no free electrons.
When there is an external electric field, the creation of dipole moments can take place in dielectrics. This is by the stretching and re-orienting of its molecules. The collective dipole moment can be defined as the net charge on the dielectric’s surface. Also, it opposes and reduces the external field. This is why they are materials that are very poor conductors of electric current.
Examples of dielectric materials are as follows:
- Porcelain (ceramic)
- Mica
- Glass
- Plastics
- Oxides of various metals.
- Some liquids and gases
- Dry air
- Distilled water
- Vacuum
Dielectrics can be easily polarised
Suppose there is an external electric field in which the atoms or molecules of a dielectric are placed. In such a case, there is pushing of nuclei within the field. This causes a high positive charge on a particular side while there is pulling of the electron clouds against it. Consequently, an increased negative charge is formed on the other side. This is a simple explanation of the process called polarisation.
In such a state, the dielectric material becomes polarised. This is why dielectrics can be easily polarised, and this can take place by two main methods:
- Stretching an atom or molecule: This causes the addition of an induced dipole moment to every atom or molecule.
- Rotation: This is an occurrence that takes place only in polar molecules. Polar molecules are those with a dipole moment that is permanent.
Types of polarisation
Below are the various types of polarisation.
- Electronic polarisation: This occurs in all atoms affected by an electric field application. There could be a possible shifting of the atom’s nucleus and its electron cloud’s centre from each other. A tiny dipole could be created with an extremely small polarisation effect.
- Ionic polarisation: In ionic solids, for example, ceramic materials, there is a symmetrical arrangement in a crystal lattice with a polarisation that is net-zero. On application of an electric field, there is the attraction of anions and cations towards opposite directions. Thereby, a relatively large ionic displacement is created.
- Dipole (or orientation) polarisation: Certain types of solids have molecular dipoles that are permanent. In an electric field, there is the rotation of such solids in the direction of the applied field. This leads to the creation of a net average dipole moment per molecule.
- Space charge (or interfacial) polarisation: In ceramics, this phenomenon takes place from extraneous charges that arise as a result of contaminants or irregular geometry. This takes place in the interfaces of the polycrystalline solids.
Conclusion
Dielectrics are materials that are very poor conductors of electric current. Here, the outer electrons are strongly attached to their nuclei. We can term these materials as insulators that contain no free electrons. Dielectrics can be easily polarised, and this can happen with two methods: stretching and rotation. There are four types of polarisation: electronic polarisation, ionic polarisation, dipole (or orientation) polarisation, and space charge (or interfacial) polarisation.
Conclusion
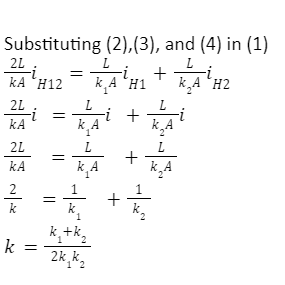
The measure of temperature is the microscopic kinetic energy of molecules and atoms that is able to transfer to neighbouring molecules and atoms in a process known as heat conduction. The rate of heat conduction varies for different objects and materials. We define a heat conduction equation as one that describes the heat flow for a body of material of known dimension and placed under a temperature difference. The coefficient of thermal conductivity is a constant that appears in this equation. Its value describes how easily heat transfer can take place for different materials. Furthermore, we discussed the applications of thermal conductivities in describing thermal conductors and insulators.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




