Hydrocarbons are the most basic organic compounds, consisting of only carbon and hydrogen. These can be found in nature in the form of everyday objects. As a result, hydrocarbons are regarded as the parents of organic compounds. All other compounds, on the other hand, are thought to be the result of functional groups being substituted for one or more hydrogen atoms.
Structure of alkynes:
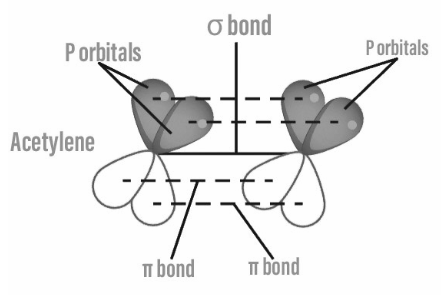
A carbon-carbon triple bond is made up of one strong bond and two weak bonds.
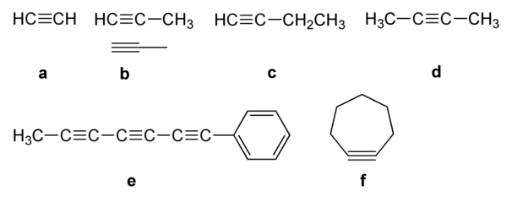
This unsaturated hydrocarbon’s overall formula is CnH2n–2, where n–2,3,4,5…… According to the IUPAC framework, alkene names have a postfix-yne, and the prefix is determined by the number of carbon particles.
Bonding in alkynes:
The H–C–C bond angles in acetylene are 180°. Alkynes have a rod-like structure due to this bond angle. Cyclic alkynes are also uncommon. It is impossible to isolate benzyne. The C–C bond distance in alkanes is much shorter (121 picometers) than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm).
With a bond strength of 839 kJ/mol, the triple bond is extremely strong. The sigma bond has a contribution of 369 kJ/mol of bond strength, the first pi bond has a contribution of 268 kJ/mol of bond strength, and the second pi-bond has a contribution of 202 kJ/mol of bond strength. Bonding is typically discussed in the context of molecular orbital theory, which recognises the triple bond as the result of s and p orbital overlap. The carbon atoms in an alkyne bond are sp hybridised in the language of valence bond theory: they each have two unhybridized p orbitals and two sp hybrid orbitals. sp orbital of each atom overlaps to form one sp–sp sigma bond.
Each p orbital overlaps on the other, resulting in two pi bonds, for a total of three bonds. Each atom’s remaining sp orbital can form a sigma bond with another atom, such as hydrogen atoms in the parent acetylene. The two sp orbitals are positioned on opposite sides of the carbon atom.
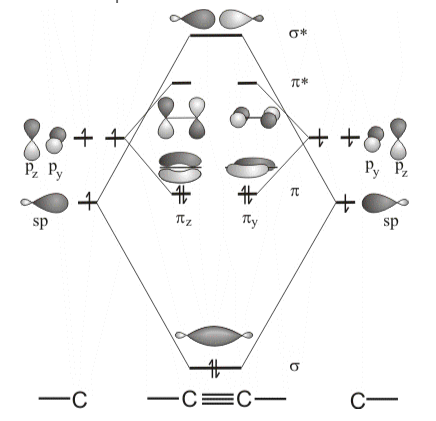
Molecular orbital theory:
The atomic or hybrid orbitals (AOs) are combined with molecular orbitals (MOs) in molecular orbital theory (MO theory) (MOs). When the sp hybrid orbitals combine, they create a bonding σ orbital and an antibonding σ * orbital. Two π and two π* molecular orbitals are formed by the four p atomic orbitals. The energy sequence is from lowest to highest MO is σ < π(y) = π(z) < π(y)* = π(z)* < σ*. In total, six electrons are available to occupy molecular orbitals, with only bonding orbitals occupied.
Isomerism in alkynes:
Alkynes have the following structural isomerism:
Chain Isomerism: Due to different carbon chain configurations, chain isomerism occurs in alkynes with five or more carbon atoms. As an illustration,
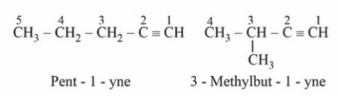
Position isomerism: In this case, the isomers differ in terms of the location of the triple bond. Butene has two isomers, for example.
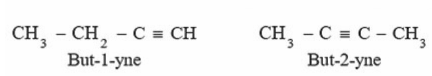
Functional Isomerism: Dienes contain two double bonds, whereas alkynes are functional isomers of dienes.
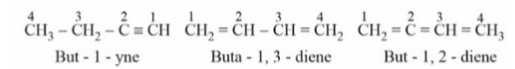
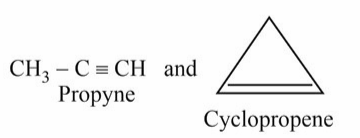
Ring Chain Isomerism: Alkynes exhibit ring chain isomerism when compared to cycloalkanes. As an illustration,
Acidic nature of Alkyne:
When it comes to the synthetic properties of alkynes, we begin with their slightly acidic nature. Alkynes are currently marginally electronegative in nature. The triply fortified carbon particles in alkynes are sp hybridised, whereas the single bond iotas in alkanes are sp3 hybridised, resulting in the difference in electronegativity. This makes it easier for them to draw in the C-H bond’s common electron pair. So, when we react with ethyne to a solid base like NaNH2, we get sodium acetylide and free hydrogen (H2) gas. Such reactions, however, will not occur in alkanes and alkenes.In the end, the hydrogen particles connected to the carbon-carbon triple bond in alkynes are slightly acidic. It should be noted that the other hydrogen particles exposing these are not acidic.
HC ≡ CH + Na → HC ≡ C– Na+ + 1/2H2
Uses of Alkyne:
- Because ethyne has a very hot fire, it is commonly used in oxyacetylene gas welding and oxyacetylene gas cutting. When ethyne is ignited with oxygen, the resulting fire has a temperature of around 3600 Kelvin.
- The superseding alkyne in acetylene is used as a fuel, with millions of kilograms produced each year by partial oxidation of gaseous petrol. A portion of these alkynes is used to make substance mixtures such as ethanoic corrosive, acrylic corrosive, and ethanol.
- Ethyne is most commonly used in the production of natural mixtures such as ethanol, ethanoic corrosive, and acrylic corrosive. It’s also used to make polymers and raw materials for them.
- Acetylene splits into two components: carbon and hydrogen. This response generates a lot of heat, which can cause the gas to light up even if there is no air or oxygen present.
- Alkynes are commonly used as starting materials in the production of a wide range of natural mixtures with mechanical significance, for example, chloroprene, vinyl chloride, and so on.
Conclusion:
Alkynes are unsaturated carbons that have three levels of security at the carbon site.Except for ethylene, which has a slight distinctive odour, all alkynes are scentless and dismal.The first three alkynes are gases, and the next eight are fluids. All alkynes above these eleven are solids.Alkynes have polar properties.
The boiling point and liquefying point of alkynes increase as their atomic design increases. The edge of boiling over increases as their subatomic mass increases. Furthermore, because of an additional bond at the carbon site, the limits of alkynes are marginally higher than those of their corresponding alkenes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




