Benzene is an organic compound consisting of six carbon atoms and six hydrogen atoms arranged in a hexagonal ring. The carbon-hydrogen bonds are strong, so Benzene does not undergo combustion but supports other chemical reactions. One of the most critical reactions involving Benzene is an electrophilic substitution. This process happens when an electron-donating atom or group replaces one of the hydrogen atoms on the ring. This article will discuss how an electrophilic substitution reaction occurs in Benzene.
Benzene is usually depicted as a hexagon with a circle inside. The alternating double and single carbon-carbon bonds give Benzene its characteristic stability.
This article will introduce the fundamental concepts underlying Nucleophilic and Electrophilic substitution in Benzene. This article will also cover Friedel-Crafts acylation and the Benzene synthesis route.
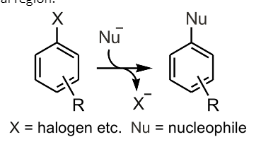
Nucleophilic Substitution in Benzene
Nucleophilic substitution in benzene occurs when a nucleophile replaces a carbon-bearing leaving group in Benzene. Since Benzene has a small nucleophile space, the OH atom must move through the ring to attack a carbon-bearing leaving group. The reaction can only occur if the nucleophile is positioned in the ring’s central region.
Benzene’s nucleophilic substitution involves a secondary addition of hydrogen to an electron-deficient ring. The substitution proceeds in several ways, but some essential features distinguish this reaction from other methods. The sH adduct is produced as an intermediate, and the X-group is removed after the nucleophilic addition. Thus, this conversion is reversible.
Mechanism of Electrophilic Substitution in Benzene
Electrophilic substitution in Benzene occurs when an electrophile substitutes a Hydrogen atom in Benzene. Electrophilic substitution in Benzene takes place in two steps, generally.
The first step of electrophilic substitution in benzene synthesis is the formation of a benzalkonium intermediate, also called a sigma complex. This intermediate is resonance stabilised and has four p electrons, making it more reactive than the original aromatic ring. However, this reaction does not proceed as quickly as an electrophilic addition to an alkene.
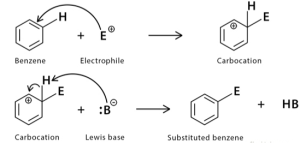
During the first step of the benzene ring electrophilic substitution, the specific substituents present in the ring can affect the orientation of the product. This is the product determining action and is the slowest step in the reaction. The rate-enhancing effects of each substituent are best understood by looking at its interactions with delocalised positive charges on intermediates.
Friedel-Crafts acylation
The Friedel-Crafts acylation in Benzene is a chemical reaction that attaches a substitute to an aromatic ring. The process involves adding a methyl or ethyl group to a benzene ring, which is an electrophilic aromatic substitution reaction. Benzene is an example of a chemical that can undergo this reaction.
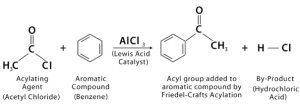
The Friedel-Crafts acylation of Benzene has been successfully performed on unsaturated benzenes using a silver nitrate catalyst. Both benzoyl chloride and acetyl chloride react with the catalyst in a simple three-step process. The Friedel-Crafts acylation can proceed as long as the ring is not deactivated.
Benzene trajectories
A single ET mediates the electrophilic substitution reaction of Benzene. The methyl cation approaches the benzene ring with a positive charge in the p orbital of its carbon. In all the trajectories, the methyl radical follows the momentum toward the benzene ring and recoils in three and a half seconds.
The benzene ring contains pi electrons, which are attracted to positive charges. This causes the pi electrons to function as a nucleophile and attack the electrophile, forming a covalent bond with the electrophile. It is essential to understand how this reaction proceeds as a result of the reactivity of the benzene ring. While the benzene ring did not react with the hydrogen atom, the electrophile added its hydrogen to the two carbons on either side of a double bond.
Electrophilic Substitution Reaction of Benzene Nitration
Benzene can be nitrated without losing its aromatic character. The electrophilic substitution reaction of benzene nitration occurs when Benzene is combined with nitric acid and sulfuric acid. Benzene and sulfuric acid form an electrophile, which reacts with the nitric acid to form a resonance-stabilised structure containing a nitrate group. In the final step, the aromatic ring of Benzene is re-formed.
C6H6 + HNO3 → C6H5NO2 + H2O
During the nitration of Benzene, a nitronium ion is produced. This results in the formation of nitrobenzene. This reaction occurs in the presence of sulfuric acid and nitric acid and occurs at a temperature of 50-degrees Celsius. Benzene is held at this temperature for about half an hour.
Conclusion
Benzene is a fascinating molecule because it undergoes electrophilic substitution reactions more readily than other hydrocarbons. This article looked at the mechanism of electrophilic substitution in Benzene. By understanding the steps involved in this process, you can better understand the properties of Benzene.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out