In chemistry and atomic physics, the most group is that the group of elements (sometimes known as the representative elements) whose lightest members area unit diagrammatical by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as organised within the periodic table of the elements.
Representative Elements On Periodic Table
To understand the question, “what are representative elements,” a touch introduction to the periodic table is important. The tabular array may be a chart that has all best-known components organised in a very definite pattern. The idea for the order of components is their number and their electron configuration. The table is in horizontal rows known as periods and vertical columns known as teams.There are eighteen groups and seven periods
The groups are named from one to eighteen from left to right
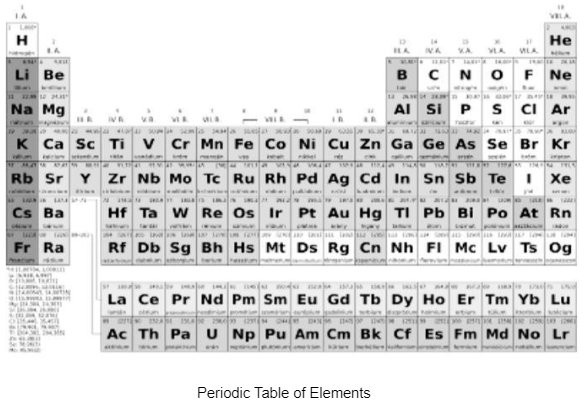
Main Group Elements
Any chemical element belonging to the s and p blocks of the periodic table is referred to as a main group element in chemistry and physics. The s-block elements are divided into two groups: alkali metals and rare earth metals (alkaline earth metals). Groups 13-18 make up the p-block elements (basic metals, metalloids, nonmetals, halogens, and noble gases). S-block elements generally have one oxidation state (+1 for group 1 and +2 for group 2). The p-block elements may have more than one oxidation state, but the most common oxidation states are separated by two units when this happens.
Helium, lithium, boron, carbon, nitrogen, oxygen, fluorine, and neon are all examples of main group elements.
Significance of the Main Group Elements
The most numerous elements in the cosmos, solar system, and on Earth are the main group elements and a few light transition metals. As a result, representative elements are frequently referred to as main group elements.
The d-block elements have traditionally not been considered main group elements. To put it another way, the transition metals in the middle of the periodic table, as well as the lanthanides and actinides below the table’s main body, are not main group elements. Hydrogen is not considered a major group element by certain scientists.
Zinc, cadmium, and mercury, according to some experts, should be included in the core group of elements. Others argue that elements from Group 3 should be included in the group. On the basis of their oxidation states, the lanthanides and actinides may be included.
Why Are They Known As Representative Elements
The representative elements have other names likewise. Initially, eight teams with A and B sub-groups divided the elements in the periodic table. group 1 is 1A, group 2, IIA. The 10 groups within the middle, when calcium, fall in teams IIIB, IVB until VIIIB, and so as IB and IIB. VIII B group has a set of 3 parts in it in every period.
Elements of sub-group A are the representative components. The transition elements are the B sub-group elements, with lanthanides and actinides in distinct rows below the main body of the table.
The representative elements also are named main group elements, s, and p block parts.
The latter is because the s orbital and also the p orbital of the energy levels get filled in their atoms.
The following reasons illustrate why these components are referred to as representative elements and also the possible definition of representative elements.
- The elements in these groups don’t have a valence shell with a full set of electrons. (Group 18 elements have.)
- They are reactive metals and nonmetals.
- The element group given cluster shows predictable chemical behaviour.
From left to right, representative components show an increase within the range of valence electrons; this results from the elements having an increasing atomic number from left to right during an amount. The element at the end of the period incorporates a crammed valence shell; this can be evident within the table image on top of and from the Lewis structures of many elements of the third period.
Representative Elements In The Periodic Table
The periodic table is a brief chart from which a lot of data may be gathered.
It divides into parts in step with the electron orbitals that get filled.
The first 3 periods are unit short, and therefore the rest area unit long. There are two periods of components isolated from the main table.
As the table is structured on electronic configuration and therefore the arrangement of components is consistent with the orbitals that get stuffed.
The beginning of an amount shows the accommodation of electrons in an exceedingly recent s orbital and therefore the finish of the amount signifies the energy being fully stuffed.
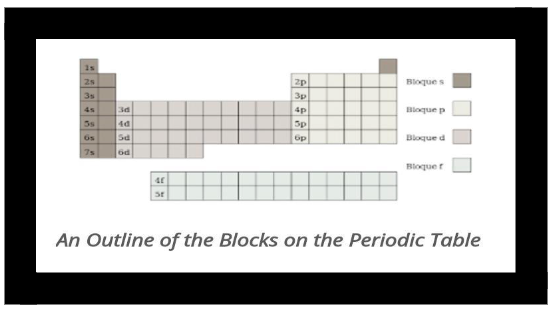
The representative components on the table area unit found on its left and right components. they’re the s block (groups one and 2) and p block components (groups thirteen to 18). For s block components, the s orbital gets filled; for p block components, the p orbital gets stuffed.
The transition components are the bulk of the table. In these components, their d orbital gets stuffed. they’re the d block components. They belong to teams three to twelve.
The two detached rows below the most table area unit components whose f orbitals area unit stuffed.The first isolated row is called lanthanides, named once the primary component, lanthanum, in this series. Lanthanides don’t belong to any cluster, as they squeeze between teams a pair of and three. The weather has their 4f orbital completed.
The actinides area unit the second isolated row, and aside from the primary four, area unit factory-made components. These components don’t belong to a gaggle -their 5f orbital yield to completion. the weather area unit named once element, the primary component within the series.
Lanthanides and actinides structure the f block components or inner transition components
Conclusion
Chemometrics suggests that using PCA and HCA to classify all representative elements into metals and non-metals based on atomic radius, first ionisation energy, electron affinity, and electronegativity is not conceivable. Metalloids are described as elements with periodic properties that fall somewhere between metals and nonmetals. As a result, metalloids must behave similarly to these two groups of molecules in various ways. The main guideline for distinguishing a metal from a non-metal, according to PCA, is that metals have a larger atomic radius but lower ionisation energy, electron affinity, and electronegativity, whereas non-metals have the opposite. Some aspects have a lot of similarities to other elements. This is due to the fact that they both have periodic qualities that are similar.
However, it’s vital to keep in mind that when other qualities like boiling point, melting point, density, and conductivity are taken into account, the behaviour of the representative elements examined using PCA and HCA can change dramatically. We hope that by implementing this technique, teachers and students will be inspired to use chemometrics to examine not only periodic table relationships, but also any chemical problem of interest. It is undeniably a fascinating and beneficial method of transdisciplinary teaching and learning.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




