Sulfuric acid (H2SO4) is a dense, colourless, oily, corrosive liquid that is also known as oil of vitriol or hydrogen sulphate. It is one of the most commercially important compounds. Sulfuric acid is created industrially by reacting water with sulphur trioxide (see sulphur oxide), which is made by combining sulphur dioxide and oxygen in a chemical reaction using either the contact or chamber processes. Fertilisers, pigments, dyes, medicines, explosives, detergents, inorganic salts and acids, as well as petroleum refining and metallurgical operations, all employ sulfuric acid in varied quantities. Sulfuric acid is the electrolyte in lead–acid storage batteries, which is one of its most well-known applications. Pure anhydrous sulfuric acid does not exist in nature due to its affinity for water. Depending on the emissions associated with individual volcanoes, volcanic activity can result in the formation of sulfuric acid, and sulfuric acid aerosols from an eruption can stay in the stratosphere for many years. Though volcanic activity is a modest contribution to acid rain, these particles can convert into sulphur dioxide (SO2), an ingredient of acid rain.
Preparation of Sulphuric Acid
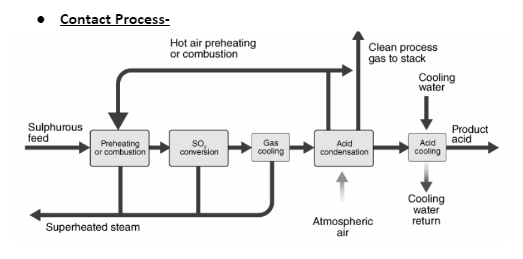
1 mole of sulphur trioxide reacts with 1 mole of water to produce one mole of sulphuric acid. This is the Contact process, which is a commercial method. In this reaction, vanadium oxide is used as a catalyst.
Lead chamber process-
The lead Chamber technique is one of the most used manufacturing methods. It produces 50 to 60 B-grade acids. In this procedure, we use wet SO2 in the presence of nitrogenous oxides (dynamic impetus). As a result, when it comes into contact with oxygen in the air, it produces sulphur trioxide. It (reaction) is given by:

Metabisulphite method-
This method involves placing metabisulfite at the bottom of a beaker and adding 12.6 molar hydrochloric acid, is a less well-known approach. As the reaction progresses, the resultant gas is bubbled through nitric acid, releasing brown/red fumes of nitrogen dioxide. When the vapours stop, the reaction is complete. This procedure does not result in an inseparable mist, which is a big plus.
Sulphur and saltpetre (potassium nitrate, KNO3) have traditionally been burned together in the presence of steam. When saltpetre decomposes, the sulphur is oxidised to SO3, which then reacts with water to form sulfuric acid.

Uses of Sulphuric Acid
- Fertilisers are made from it.
- It’s used in the steel and iron industries. In the industrial sector, it’s also used to remove rust from steel and iron.
- It’s used in the chemical manufacturing business, for example. Phosphoric acid, hydrochloric acid, nitric acid, sulphate salts, synthetic detergents, dyes and pigments, explosives, pharmaceuticals, and ammonium sulphate are among the products made from it.
- It’s used in the oil-processing industry.
- It aids in the conversion of cyclohexanone oxime to caprolactam by acting as a catalyst. In the manufacturing of nylon, this reaction is used.
- It is used in the manufacture of batteries. Sulphuric Acid is used as an electrolyte in lead-acid batteries. It’s suitable for usage in storage batteries. It’s also known as vehicle battery acid because it’s found in a car battery.
- Up to 50% of the produced liquid is utilised to make phosphoric acid, which is used to make phosphoric acid fertilisers. It’s also utilised in the creation of pigments, paints, plastics, and metals like copper and zinc; 5% of the acid produced is used in the production of fibres, explosives, and pharmaceuticals, as well as the leather and petroleum industries. It’s used in almost every industry.
- It is a highly hazardous substance that should be handled with extreme caution. Acid drain cleaners contain it. It can be used to remove tissue paper due to its high drainage.
- It is employed as a catalyst in the production of nylon. In the manufacturing of HCl, it is used in the Mannheim process.
- In the manufacturing of chemotherapeutic medicines, it is used to damage the DNA of cancer cells. It’s also found in ointments that are used to treat a variety of skin ailments.
Characteristics of Sulphuric Acid
- Pure sulfuric acid has a specific gravity of 1.84 at 288 K and is colourless, odourless, high density, greasy liquid.
- At all concentrations, it is extremely soluble in water.
- It contains 97.3 percent acid and boils at 611 degrees Celsius. As a result, boiling cannot increase the concentration of aqueous sulfuric acid above 97.3 percent.
- In the humid air, it will suffocate.
- Sulphuric acid can cause serious burns if it comes into contact with the skin.
- This substance is very corrosive, reactive, and water soluble. It has a strong oxidising power, making it a very effective oxidizer.
- It has a low volatility level. As a result, it facilitates the formation of more volatile acids from their opposing salts.
CONCLUSION
Sulfuric acid (H2SO4) is a transparent, oily mineral acid with a boiling point of 554 degrees Fahrenheit (290 degrees Celsius). The mineral sulphur is used to make it. Sulfuric acid accounts for more than 80% of all sulphur generated. The acid’s strength and high boiling point make it valuable in the production of other acids. Sulfuric acid dissolves a wide range of metals on its own; when combined with hydrochloric acid (a mixture known as aqua regia), it may dissolve gold and platinum. Because the reaction produces a lot of heat, diluting sulfuric acid with water can be harmful. The acid must be introduced to the water, not the other way around, to avoid explosive spattering. Scientists most likely discovered how to produce sulfuric acid after the year 1000. Alchemists first characterised sulfuric acid around 1300. Sulfuric acid was given the name oil of vitriol by alchemists because of its caustic properties. Andreas Libavius (c.1540-1616), a German alchemist, published clear instructions on how to produce sulfuric acid and other chemical substances in 1595. During the Middle Ages, when sulfuric acid and other strong acids became widely available, they ushered in an experimental revolution, allowing alchemists to quickly disintegrate substances without the use of high temperatures.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




