Joseph Priestley discovered ammonia gas in 1771 when he combined ammonium and lime and termed it Alkaline Air. He was the first person to discover ammonia gas.
It may be found in trace amounts in the atmosphere at certain concentrations. In areas where animals decay, the amount of rotting matter in the air is more than anticipated. It may also be found in nature in the form of salts.
Ammonia (NH3) is a nitrogen and hydrogen molecule that is necessary for life. It is formed as a result of the constant disintegration of vegetative and animal bodies. The death and decay of animals and plants causes the nitrogen compounds present in them to degrade, resulting in the production of ammonia as a result. Ammonia, on the other hand, may be found in the soil in the form of ammonium salts.
Ammonia
Ammonia is produced in soil by bacterial processes as part of the nitrogen cycle in the environment.In addition to decomposing organic matter like plants and animals, ammonia is also produced naturally. Most of the ammonia produced by the industrial sector is used for fertiliser in agriculture.It is also used as a refrigerant gas, as a purification gas in water supplies, and as a chemical for making plastics, explosives, textiles, pesticides, dyes, and other products. Cleaning solutions with this ingredient are commonly used in the home and industrial environment.The ammonia in household cleaning solutions is primarily derived from a gas, which is added to water. The solution can consist of between 5 and 10% ammonia. Industrial ammonia solutions can have concentrations up to 25% and can be corrosive.
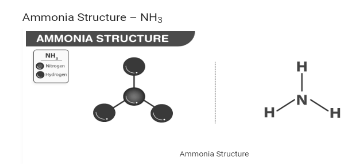
Structure of Ammonia
Preparation of Ammonia – NH3
By heating an ammonium salt such as ammonium chloride NH4Cl in the presence of a strong alkali such as sodium hydroxide or calcium hydroxide, it is possible to readily convert it to ammonia in the laboratory.
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3(g)
Warming concentrated ammonium hydroxide can also produce the gas.
Haber Process, under pressure and with a catalyst, is the most common method of commercially producing ammonia.
Ammonia Uses
- Fertilisers are used to increase crop yields by using it.
- As a household cleaner, NH3 is used to clean stainless steel and glass by mixing it with water.
- It is used in antimicrobial food products
- It is used in the fermentation industry
- It is used to cool and keep products cool
- It is used to maintain the pH of the fermentation process
- Pollutants like nitrogen oxide, which are emitted by diesel engines, can be neutralised by it.
- Rocket engines use it as fuel
- It is used in the textile industry
- It is used to produce synthetic fibres such as rayon and nylon
How can people be exposed to ammonia?
Inhalation of gases or vapours exposes most people to ammonia. Among the sources of exposure to ammonia are cleaning products, natural sources, and cleaning products. Ammonia is frequently used in farms, factories, and commercial locations, which means that accidental releases or terrorist attacks may also lead to exposure.
The gas rises and dissipates, so it does not settle in low-lying areas since anhydrous ammonia gas is lighter than air. Anhydrous ammonia gas, on the other hand, forms heavier vapours when it comes into contact with moisture (as in the presence of a high relative humidity). It is possible for people to become exposed to these vapours as they spread across the ground or go into low-lying areas with poor airflow.
Immediate health effects of ammonia exposure
Inhalation: In addition to being irritating, ammonia is also corrosive.The nose, throat and respiratory tract burn instantly when exposed to high concentrations of ammonia in the air.There can be respiratory distress or failure as a result of breathing difficulty and bronchial and alveolar edema. Coughing and throat irritation are caused by inhaling lower concentrations. Aside from the potent odour of ammonia, it also causes olfactory adaptation and fatigue, which reduces awareness of prolonged exposure at low concentrations.
Since children have greater lung areas-to-body weight ratios and larger minute volumes-to-weight ratios, they may receive a higher dose due to higher concentrations of ammonia vapour. Due to their shorter heights and the higher concentrations of ammonia vapour near the ground, they may also be exposed to higher concentrations of ammonia than adults in the same location.
Skin or eye contact: Ammonia may irritate the skin or eyes at low concentrations of air or solution. More severe injuries and burns may occur at higher concentrations. Industrial cleaners containing concentrated ammonia solutions can cause chemical burns, permanent eye damage, and blindness due to the corrosive nature of the solution. Up to a week after the exposure may seem to be too late to realise how serious the eye injury is.
Ingestion: By swallowing ammonia solution, users are exposed to high concentrations of ammonia, which causes corrosive damage to their mouths, throats, and stomachs. Systemic poisoning is rarely caused by ingestion of ammonia.
Ammonia exposure treatment
The effects of ammonia can be treated, but there is no antidote for ammonia poisoning. Most people recover from the effects of ammonia. A great deal of water should be used to decontaminate the skin and eyes immediately. Patients with asthma are typically treated with supportive measures, including humidified oxygen, bronchodilators, and airway management.Milk or water is administered to dilute ingested ammonia.
Conclusion
As a precursor for amino acid and nucleotide synthesis, ammonia is essential for many biological processes. Ammonia is a key component of the nitrogen cycle and is produced in soil by bacteria.
Besides ammonia, a great number of chemical compounds and many synthetic products have been manufactured on a large scale through the industrial production of ammonia. Ammonia cannot be used in a conventional engine without losing power. In spite of this, ammonia remains a significant alternative fuel for internal combustion engines, since it is a carbon-free fuel that can reduce carbon emissions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






