Other mineral acids are more corrosive than phosphoric acid. Many materials, at least at low temperatures, show useful resistance to phosphoric acid. Corrosion rates are often accelerated by temperature and acid contaminants. At concentrations up to 55 percent and temperatures above the boiling point, zirconium resists assault in phosphoric acid. The corrosion rate of zirconium increases with temperature above 55 percent phosphoric acid. Dilute acid at high temperatures would be the most interesting area for zirconium. Anodic polarisation curves of zirconium in phosphoric acid at near-boiling temperatures. As concentration rises, the passive range gradually decreases, whereas the passive current gradually rises. Zirconium appears to passivate more slowly in phosphoric acid than in other mineral acids.
Chemical Reactivity of Phosphoric Acid
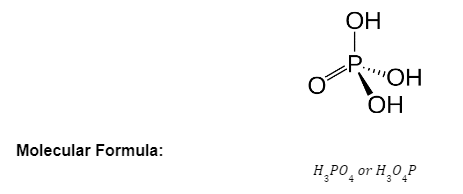
Phosphoric acid (H3PO4), also known as orthophosphoric acid, It’s the most important phosphorus oxygen acid, and it’s used to generate phosphate salts for fertilisers. It’s also employed in dental cements, albumin derivative manufacturing, and the sugar and textile sectors. It is used in food goods as an acidic, fruity flavouring(NaH2PO4)
Structure of Phosphoric Acid:
Health Hazards:
Burns on the lips and mouth, a foul acrid taste, severe gastrointestinal irritation, nausea, vomiting, bloody diarrhoea, difficulty swallowing, severe stomach pains, thirst, acidemia, problems breathing, convulsions, collapse, shock, and death (United States Coast Guard, 1999)
Reactivity Profile:
PHOSPHORIC ACID is a kind of phosphoric acid that occurs naturally in the When it comes to bases, it reacts in an exothermic way. May react with active metals to release hydrogen, a flammable gas, including structural metals like aluminium and iron. Certain kinds of organic compounds can be polymerized using this agent. When it comes into contact with cyanide compounds, it produces hydrogen cyanide gas. When dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulphides, and powerful reducing agents come into contact, they can form flammable and/or poisonous fumes. Nitromethane creates an explosive combination. Sodium tetrahydroborate causes a strong reaction. Stainless steel can corrode and produce explosive hydrogen gas in the presence of chlorides. When heated to breakdown, it releases hazardous and unpleasant phosphorus oxide fumes [Lewis, 3rd ed., 1993, p. 1029].
Phosphoric Acid and Hydrogen
Phosphoric Acid: H3O4P, or an aggregation of orthophosphoric acid molecules, is a weak, colourless, odourless, and inorganic acid. It’s recognised as a divalent cation binding agent in chemistry. It’s used as a cleansing and roughening agent, as well as an acidifying and pH regulating agent, in therapeutic operations. It is solid at room temperature, but beyond 108°F (42.35°C), it is usually 85 percent an aqueous solution. It can be utilised in the fabrication of surface finishing agents (paints and coatings) as well as as a corrosion inhibitor in the industry.
Hydrogen: a nonmetallic gaseous chemical element with the atomic number 1 that is the simplest and lightest of the elements and is employed in the processing of fossil fuels and the synthesis of ammonia.
Conclusion
Phosphoric acid is a triprotic acid that exists as a thick liquid. Orthophosphoric acid is another name for it. Both humans and laboratory animals’ skin, eyes, and other mucous membranes are irritated or corroded by it. Its salts, on the other hand, have a far reduced irritancy potential. When mice were exposed via inhalation, moderate toxicity was detected. Although phosphate salts have been shown to increase the action of recognised carcinogens, phosphoric acid is neither genotoxic nor carcinogenic. Irrigation or water flushing are commonly used to treat exposures. Phosphoric acid has attracted a lot of attention as a food ingredient for various cola drinks, but there’s a lot of debate over whether it’s safe. When it comes to pollution of the aquatic environment, the pH of the water is the most important factor to consider in terms of the effects on native flora and fauna. There is no evidence of bioamplification or bioaccumulation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




