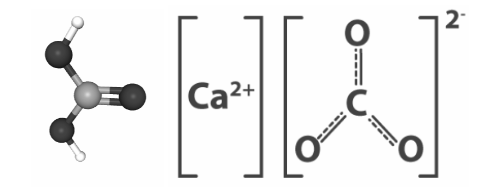
The chemical compound calcium carbonate has the formula CaCO3. It is the principal component of eggshells, gastropod shells, shellfish skeletons, and pearls and is found in rocks as the mineral calcite and aragonite (most famously as limestone, which is a form of sedimentary rock consisting primarily of calcite). Calcium carbonate is the active ingredient in agricultural lime, and it is created when calcium ions in hard water react with carbonate ions to form limescale. However, taking too much of it might cause hypercalcemia and digestive issues. It is used as a calcium supplement and as an antacid in medical practice.
Structure of Calcium Carbonate
Calcium carbonate is an odorless, non-toxic chemical that occurs naturally as a white mineral in limestones, chalks, and marbles.
Calcium Carbonate has the following structure:
Reactivity of Calcium Carbonate with oxygen
Calcium carbonate is mined and processed largely from a variety of natural mineral sources. It can also be manufactured chemically by mixing quicklime (calcium oxide, CaO) with water to get calcium hydroxide (Ca(OH)2), which is then treated with carbon dioxide to generate calcium carbonate salt.
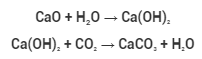
It’s made by passing carbon dioxide gas through calcium hydroxide on a big scale (otherwise called slaked lime). However, if there is an excessive amount of carbon dioxide present, soluble calcium hydrogen-carbonate is formed.
Application of Calcium Carbonate
- Calcium carbonate is primarily used in the paper and pulp manufacturing sectors. Additionally, it can be used as a pigment and filter, allowing for the manufacture of a whiter, higher-quality pigment than is achievable with other minerals.
- Calcium carbonate is a filler material used in the construction industry to improve the appearance and durability of concrete and to purify metals for use in construction applications.
- Calcium carbonate is also used in fertilizers to provide calcium to plants and to stabilize the soil’s pH.
- Additionally, it can be used as a vitamin supplement and as an ingredient to food products for people and farmed animals.
- Calcium carbonate is used to remove pollutants and acidity from water and wastewater treatment plants.
- A common application for calcium carbonate is as an effective dietary calcium supplement, antacid and phosphate binder, or as a base material for pharmaceutical tablets. It can also be found on the shelves of many grocery stores in products such as baking powder, toothpaste, dry-mix dessert mixes, dough, and wine, among other things.
Other noteworthy points
If you have been prescribed this prescription or compound, keep all scheduled appointments with your doctor so that your response to the substance can be monitored. Additionally, do not allow anyone else to take your medication.
Additionally, it is critical for you to maintain a documented list of all medications you are taking, including prescription and nonprescription (over-the-counter) medications, as well as any items you are using, such as minerals, vitamins, or other dietary supplements. Additionally, you should bring this list with you whenever you visit a doctor or are admitted to a hospital. Additionally, it is critical to have this with you in case of an emergency.
As part of her extravagance, Cleopatra is claimed to have dissolved pearls made of calcium carbonate in vinegar and drank the solution.
Stalagmites and stalactites form when calcium bicarbonate dissolved in groundwater reaches the ceiling of a cave and releases carbon dioxide (CO2). Calcium bicarbonate is transformed to calcium carbonate when it absorbs CO2, forming a stalactite on the roof. When calcium bicarbonate does not absorb carbon dioxide before pouring down and collapsing on the floor, calcium carbonate accumulates and forms a stalagmite.
Calcium Carbonate is used to neutralize the acidity of lakes and other bodies of water, to filter water and wastewater, and to treat waste gasses by eliminating sulfur and nitrogen oxides that contaminate the air.
CONCLUSION
Calcium carbonate is a chemical substance that has no odor. It is a calcium source that is insoluble in water. It’s a calcium carbonic salt that’s mostly found in rocks.
Calcite, Vaterite, and Aragonite are examples of pure calcium carbonate minerals. Snail shells, egg shells, oyster shells, and other biological sources of calcium carbonate It’s most commonly used as an antacid or a calcium supplement. The PH value is approximately 9.91. Limestone is its common name.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






