When an element occurs in more than one crystalline form, such forms are referred to as allotropes; diamond and graphite are the two most prevalent allotropes of carbon. A Diamond’s crystal structure is an endless three-dimensional array of carbon atoms, each of which creates a structure with equal angles between its bonds. When the ends of the bonds are joined, the structure resembles that of a tetrahedron, a four-faced three-sided pyramid (including the base). Each carbon atom is covalently connected to four other carbon atoms at the tetrahedron’s four corners. The length of the link between two carbon atoms is 1.54 108 cm, which is referred to as the single-bond length.
Allotropic forms of Carbon
Carbon is one of the few elements with a large variety of allotropic forms due to its propensity to have varying oxidation states or coordination numbers. Another issue is carbon’s capacity to catenate. As a result, distinct allotropes of carbon are formed.
Allotropes of Carbon
- Diamond: It is an exceptionally hard, transparent crystal having a tetrahedral structure of carbon atoms. This allotrope of carbon is a poor conductor of electricity but a good conductor of heat.
- Lonsdaleite: Also known as hexagonal diamond.
- Graphene is the fundamental structural component of allotropes, nanotubes, charcoal, and fullerenes.
- Q-carbon: These carbon allotropes are ferromagnetic, robust, and have a more dazzling crystal structure than diamonds.
- Graphite is a soft, black, flaky substance that conducts electricity somewhat well. The carbon atoms are linked in hexagonal lattices (graphene), which are subsequently stacked to form sheets.
- Acetylenic carbon with a linear structure (Carbyne)
- Amorphous carbon
- Fullerenes, such as Buckminsterfullerene, are sometimes referred to as “buckyballs,” such as C-60.
- Carbon nanotubes: Carbon allotropes have a spherical nanostructure.
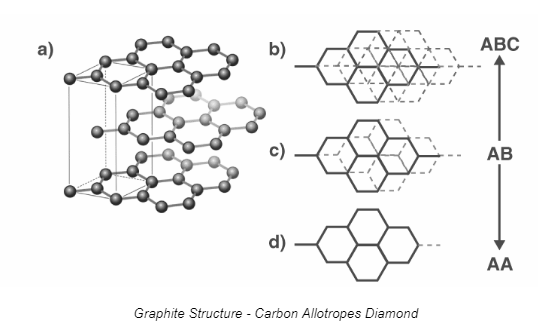
Graphite
It is a pure form of carbon. This carbon allotrope is formed of hexagonally organised flat two-dimensional layers of carbon atoms. It is supple, dark, and slick solid. Graphite retains this feature due to its ease of cleavage between the layers.
Each C atom in each layer is covalently bonded to three other C atoms through a C-C covalent connection. Each carbon atom in this structure is sp2 hybridised. As the fourth bond, a pi bond is formed. Due to the delocalization of the electrons, they are mobile and capable of conducting electricity.
Graphite is available in two forms: α and ß.
The layers in α form are placed in the order ABAB, with the third layer directly above the first.
The layers are ordered as ABCABC in the ß form.
Properties of Graphite:
- Because the layers are layered on top of one another, this carbon allotrope acts as a lubricant;
- It also has a metallic sheen, which aids in the conductivity of electricity. It is an excellent conductor of heat as well as electricity.
- One of the most important qualities of graphite is that it may be used as a dry lubricant in machines that operate at elevated temperatures and cannot be lubricated with oil.
- Graphite is used to manufacture crucibles that are inert to both dilute acids and alkalis.
Carbon Allotrope (Graphite) Structure:
Graphite has an unusual honeycomb-layered structure. Each layer is composed of carbon atoms arranged in planar hexagonal rings with a carbon-carbon bond length of 141.5 picometers.
Three carbon atoms create sigma bonds, whereas the fourth carbon atom produces a pi-bond. Vander Waal forces hold the graphite layers together.
Diamond
It is the finest form of carbon in its crystalline state. It has a number of carbon atoms that are tetrahedrally connected. Each tetrahedral unit is composed of carbon bound to four carbon atoms that are itself connected to other carbons. This results in the formation of a carbon allotrope with a three-dimensional arrangement of C-atoms.
Each carbon atom is sp3 hybridised and makes covalent connections with four other carbon atoms at the tetrahedral structure’s four corners.

The reason behind the hardness of Diamond:
It is difficult because shattering a diamond crystal necessitates the rupture of several strong covalent connections. It is not easy to break covalent connections. As a result of this feature, this carbon allotrope is the hardest element on the planet.
Diamond’s Physical Properties include the following:
- It is extremely hard
- It is extremely flammable and has a very high melting point.
- It has a high relative density
- It is transparent to X-rays
- It has a high refractive index
- It is an inefficient conductor of electricity
- It has excellent heat conduction properties.
- It is insoluble in all solvents
Additional Carbon Allotropes
Buckminsterfullerene
Buckminsterfullerene (C-60) is also a kind of carbon allotrope. Due to the cage-like structure of fullerene, it resembles a football.
Fullerenes
They are spheroidal molecules with the formula C2n, where n equals 30. These carbon allotropes can be synthesised by laser evaporating graphite.
Fullerenes are more soluble in organic solvents than diamonds, which is in contrast to diamonds. C60 fullerene is referred to as ‘BuckminsterFullerene’. sp2 hybridization occurs between the carbon atoms.
Silicates
Silicates are formed when alkali oxides are fused with SiO2. They are composed of distinct tetrahedral components. Silicon has undergone sp3 hybridization. Carbon allotropes are classed according to their structures.
- Orthosilicates: These materials are composed of distinct SiO4 units. For instance, Willemite (ZrSiO4).
- Pyrosilicate: A compound composed of two units connected by an oxygen atom. Si2O76- is the simplest ion of this kind. For instance, Thortveitite (Sc2[Si2O7]).
- Cyclic Silicates: These compounds have two oxygen atoms in common. At the moment, only two ions are known: Si3O96- and Si6O1812-. For instance, beryl is composed of the chemical formula Be3Al2Si6O18.
- Chain Silicates: When the units are linked linearly, chain silicates are formed. They are classified into two categories:
- Metasilicates: Each tetrahedral unit contains two oxygen atoms, forming a carbon allotrope with a single chain. As an example, consider Spodumene NaAl (SiO3)2.
- Amphiboles: When two linear chains are connected, the amphibole’s carbon allotrope is formed. The parallel chains are kept together by the oxygen atoms being shared. Asbestos, for example, CaMg3O (Si4O11).
- Two-dimensional silicates: When three oxygen atoms are shared, a two-dimensional silicate is formed. For instance, mica.
- Silicate three-dimensional network: When all of the oxygen atoms are shared, a three-dimensional network forms. For instance, zeolites.
Conclusions
Carbon’s valence allows for the formation of several allotropes (structurally distinct forms of the same element). Diamond and graphite are two well-known forms of carbon. Numerous further allotropes have been found and studied in recent decades, including ball forms like buckminsterfullerene and sheets like graphene. Carbon nanotubes, nanobuds, and nanoribbons are examples of larger-scale structures. At extremely high temperatures or pressures, other strange forms of carbon occur. According to the Samara Carbon Allotrope Database, around 500 potential three-period allotropes of carbon are known at the moment (SACADA).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




