An alkane is an acyclic saturated hydrocarbon in organic chemistry, commonly known as paraffin (a historical term with several meanings). An alkane is made up of hydrogen and carbon atoms that are organised in a tree structure with single carbon–carbon bonds. The chemical formula of alkanes is CnH2n+2. The alkanes range in complexity from the simplest example of methane (CH4) (often referred to as the parent molecule) to arbitrarily massive and complicated compounds like pentacontane (C50H102) or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (C14H30).
In an alkane, each carbon atom has four sigma bonds (C–C or C–H), and each hydrogen atom is attached to one of the carbon atoms (in a C–H bond). The carbon skeleton, also known as the carbon backbone, is a molecule’s longest string of interconnected carbon atoms. An alkane’s size is determined by the number of carbon atoms in it.
Alkanes are mostly obtained commercially from petroleum (crude oil) and natural gas.
Structure of Alkanes
CnH2n+2 is the general formula for alkanes. An alkane containing 2(n) carbon atoms, for example, will have 6 (2n + 2) hydrogen atoms. Sigma bonds unite their neighbouring atoms, forming tetrahedral centres surrounding the carbon atoms. Because these are all single bonds, all connections are free to rotate. Each hydrogen atom is connected to a carbon atom by four bonds (either C-H or C-C bonds) (H-C bonds).
Alkanes, commonly known as paraffins, are a kind of hydrocarbon that is entirely hydrogen-saturated. They have the carbon and hydrogen which are connected covalently since they have no double or triple bonds in their carbon skeletons. Alkenes and alkynes, on the other hand, have double and triple bonds and are classified as unsaturated hydrocarbons.
It’s possible that the structural formula for alkanes might be stated in a more compact manner. Pentane, for example, has three groups of CH2 methylene in the middle section of its structural formula. It’s possible to join them together and write down the structural formula. Below are the first five alkane formula with an unbranched chain:
Organic compound formulas include information on the compounds’ various characteristics and structures. The number and kinds of atoms in a molecule of a substance are determined by molecular formulae, such as the one for octane. C8H18 is a molecular formula that may be used for a variety of alkanes, each with its own set of physical, chemical, and toxicological characteristics. Structural isomers of a chemical are compounds having the same molecular formula but different structural formulae. Alkanes may now be used to extract a wide range of chemical molecules. Furthermore, many key components of organic compounds have one or more alkane groups. Many organic compounds have names derived from alkanes as a result of these reasons.
Types of Alkanes and Examples
Alkanes come in three different chemical structures: linear, branching, and cyclic. We’ll describe each kind and provide examples in this section.
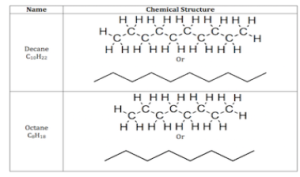
The carbons in linear alkanes are bound together in a snake-like chain structure. Some examples of linear alkanes are included in the table below. Let’s look at decane as an example. There are two ways to depict the chemical structure. It can be represented with all of the carbon and hydrogen atoms visible, or as a chain (without the hydrogen atoms), with each edge representing a carbon atom.
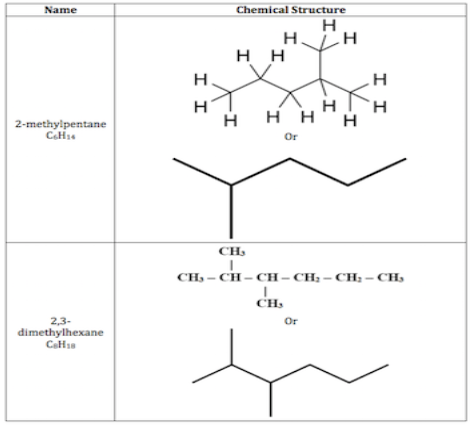
Branched alkanes are produced from the combination of linear alkanes, but their chemical structure is branched with one or more alkyl groups instead of merely a straight chain. The group of carbon and hydrogen atoms connected to an alkane molecule is known as an alkyl group.
Cyclic alkanes, which are known as cycloalkanes, are made up of hydrogen and carbon atoms that are bound together by single bonds, with the carbon atoms forming a ring or loop.
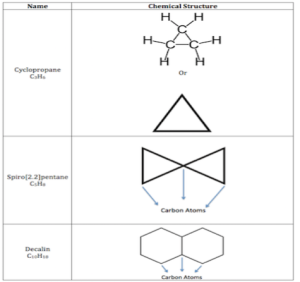
Molecular Formula
Carbon atoms in alkanes can form straight chains, branched chains, or rings, much like they do in other organic compounds. Straight-chain alkanes, branched-chain alkanes, and cycloalkanes are the three kinds of alkanes.
The following is the typical molecular formula:
- For straight-chain alkanes and branched-chain alkanes: CnH2n+2
- CnH2n is the symbol for cyclic alkanes.
Alkanes’ Nomenclature
Following the hydrocarbon prefixes, alkanes are given the suffix “-ane.” Methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), pentane, and other gases are included in the series. The prefixes “hex-,” “hept-,” “oct-,” “non-,” and “dec-“ are used for carbon chains with 6, 7, 8, 9, and 10 atoms, respectively.
The four bonds generated by carbon in larger molecular weight compounds allow for a variety of carbon Skeleton modifications. Isomers are different types of molecules that have the same chemical formula. The linear, unbranched forms of these alkanes are designated by the prefix “n-,” which stands for “normal.”
Conclusion
- Alkanes are saturated aliphatic hydrocarbons
- Alkanes have an open-chain structure with single covalent bonds connecting carbon atoms
- All four carbon valencies are supplied by four atoms or a group of atoms in alkanes
- Alkanes are also known as paraffin because they are resistant to chemical reactions (Latin: parium means little, affinis means affinity or reactivity)
- General formula of alkanes is: CnH2n+2.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




