G3(MP2) calculations producing the enthalpy of hydrogenation Delta(hyd)H(298) of 1,3-butadiene suggest that it is not stabilised by the conjugated configuration of its triple bonds, in contrast to 1,3-butadiene, the textbook example of “conjugation stabilisation.” In light of CAS-MCSCF calculations on a 1,3-butadiene and 1,3-butadiyne, differences between ethylene and acetylene pi bonds are investigated
1, 3- butadiyne
The chemical compound Diacetylene (also known as Butadiyne) has the formula C₄H₂. It is the most basic chemical with two triple bonds. It is the first of a series of polyynes that are theoretically interesting but not practical.

Occurrence
Diacetylene’s unique vibrational spectrum has been found in the atmosphere of Titan and the protoplanetary nebula CRL 618. It is thought to be caused by a reaction between acetylene and the ethynyl radical (CH2•) formed by photolysis of acetylene. This radical can then target the acetylene triple bond, causing it to react quickly even at low temperatures. Diacetylene has been discovered on the Moon as well.
Preparations
Dehydrohalogenation of 1,4-dichloro-2-butyne with potassium hydroxide (in alcoholic media) at 70°C can yield this chemical.
ClCH₂C≡CCH₂Cl + 2 KOH → HC≡C−C≡CH + 2 KCl + 2 H₂O
The Hay coupling of (trimethylsilyl)acetylene can yield the bis(trimethylsilyl)-protected derivative.
2 Me₃Si−C≡CH → Me₃Si−C≡C−C≡C−SiMe₃.
Chemical Formula Description
The molecule 1,3-Butadiyne has a total of 6 atoms (s). There are 2 hydrogen atoms and 4 carbon atoms in this molecule (s) As a result, the chemical formula for 1,3-Butadiyne can be expressed as C₄H₂
The molecular formula for 1,3-Butadiyne, as shown above, indicates the numbers of each type of atom in a molecule without structural information, as opposed to the empirical formula, which offers numerical proportions of atoms of each type.
Stoichiometry in chemical equations, or the computation of relative quantities of reactants and products in chemical reactions, is based on the chemical formula above. In a chemical reaction, the quantity of each element in the chemical formula does not change, according to the law of conservation of mass. As a result, depending on the chemical formula, each side of the chemical equation must represent the same quantity of any single element.
Butadiene:
The organic molecule 1,3-butadiene has the formula (CH₂=CH)₂. It’s a colourless gas that condenses quickly into a liquid. It is used as a predecessor to synthetic rubber in industry. The molecule is made up of two vinyl groups joined together. It’s the most basic conjugated diene there is.
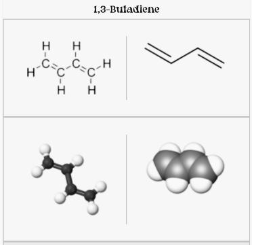
Despite the fact that butadiene degrades quickly in the atmosphere, it is nevertheless detected in the ambient air in urban and suburban regions as a result of frequent automobile emissions.
The isomer 1,2-butadiene, which is a cumulated diene with the structure H₂C=C=CHCH₃, is also known as butadiene. This allene isn’t used in any industry.
Production
Extraction From C4 Hydrocarbons
Butadiene is created as a byproduct of the steam cracking process used to make ethylene and other alkenes in the United States, Western Europe, and Japan. When aliphatic hydrocarbons are mixed with steam and cooked to extremely high temperatures (typically over 900°C), hydrogen is released, resulting in a complex combination of unsaturated hydrocarbons, including butadiene. The amount of butadiene produced is determined by the hydrocarbons that are utilised as feed. When light feeds, such as ethane, are cracked, the main product is ethylene, but heavier feeds produce heavier olefins, butadiene, and aromatic hydrocarbons.
Extractive distillation with a polar aprotic solvent such as acetonitrile, N-methyl-2-pyrrolidone, furfural, or dimethylformamide is used to separate butadiene from the other four-carbon hydrocarbons produced in steam cracking, from which it is then stripped by distillation.
From Dehydrogenation Of N-Butane
Normal butane can also be catalytically dehydrogenated to yield butadiene (n-butane). In Houston, Texas, the first postwar commercial factory opened in 1957, producing 65,000 tonnes of butadiene per year. Prior to that, the Rubber Reserve Company, a branch of the US government, built numerous plants in Borger, Texas, Toledo, Ohio, and El Segundo, California, as part of the US Synthetic Rubber Program, to make synthetic rubber for the war effort. There was a total capacity of 68 KMTA (Kilo Metric Tons per Annum).
The Houdry Catadiene technique, which was discovered during World War II, is now used to commercially generate butadiene from n-butane. This requires processing butane at high temperatures over alumina and chromia.
From Butenes
Catalytic dehydrogenation of typical butenes can also yield 1,3-butadiene. During World War II, the United States Synthetic Rubber Program (USSRP) adopted this approach. The technique was far less expensive than using alcohol or n-butane, but it had to compete with aviation fuel for available butene molecules (butenes were plentiful thanks to catalytic cracking). In Baton Rouge and Lake Charles, Louisiana; Houston, Baytown, and Port Neches, Texas; and Torrance, California, the USSRP built many plants. A total of 275 KMTA was produced per year.
In the 1960s, a Houston business known as “Petro-Tex” developed a method of oxidative dehydrogenation employing a proprietary catalyst to create butadiene from conventional butenes. It’s unknown if or not this technique is used commercially.
For Laboratory Use
Because 1,3-butadiene is a gas, it is difficult to employ in the laboratory. Its production from nongaseous precursors has been optimised in the laboratory. The retro-Diels-Alder reaction of cyclohexene can create it. In the laboratory, sulfolene provides a useful solid storable source of 1,3-butadiene. When heated, the diene and sulphur dioxide are released.
Conclusion
The majority of butadiene is used to generate synthetic rubbers for tyres, grommets, and elastic bands. Polymerisation, a process in which small molecules (monomers) are joined to form larger ones, is used to convert butadiene to synthetic rubbers (polymers). Polymerization of butadiene results in polybutadiene, a highly soft, nearly liquid substance. Copolymers are created by polymerizing butadiene and other monomers, thus they are more valuable. Butadiene and styrene polymerization and/or acrylonitrile polymerization, such as acrylonitrile butadiene styrene (ABS), nitrile-butadiene (NBR), and styrene-butadiene polymerization (SBR). Depending on the monomer ratios utilised in their manufacture, these copolymers are tough and/or elastic. SBR is the most often used substance in the manufacture of automotive tyres. Butadiene is used to make precursors for various synthetic rubbers. Chloroprene is one of them.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




