This effect has a long duration and is commonly shown by an arrow symbolise on the bonding material itself. Experiments on relative inductive effects have been conducted with increasing order of +I effect or decreasing order of -I effect, as shown in the following: Hydrogen is represented by the letter H, Deuterium by the letter D, and Tritium by the letter T. These are all hydrogen isotopes. The Hammett equation, which defines the relationship between reaction rates and equilibrium constants with respect to substituents, can be used to quantify inductive effects in a variety of situations. Atoms or functional groups that are electronegative in comparison to hydrogen, such as the halogens, oxygen, nitrogen, and so on, may exhibit a negative inductive effect, which is the opposite of what hydrogen does
Body
This phenomenon is caused by the polarisation of bonds within a molecule or ion, and it is an electronic effect. The tendency of functional groups to release electrons is referred to as the positive inductive effect. For instance, alkyl, aryl, metals, and so on. The electron accepting tendency of functional groups is referred to as the negative inductive effect. With relation to hydrogen, relative inductive effects have been measured experimentally in rising order of +I effect or decreasing order of -I effect, as follows: {-NR3+>-NH3+>-NO2>-SO2R>-CN>-SO3H>-CHO>-CO>-COOH>-COCl>-CONH2>-F>-Cl>-Br>-I>-OH>-OR>-NR2>-NH2>-C6H5>-CH=CH2>-H}
hydrogen isotopes
H is Hydrogen, D is Deuterium, and T is Tritium, in increasing order of +I effect. All are hydrogen isotopes. The distance between the substituent group and the primary group that reacts affects the strength of the inductive action; the longer the distance, the weaker the impact. The inductive effect is the result of a sigma electron shift towards a more electronegative atom, causing one end to become positively charged and the other to become negatively charged “– The I effect is a long-lasting effect that is usually indicated on the bond by an arrow.”
Hammett equation
The Hammett equation, which defines the connection between reaction rates and equilibrium constants with regard to substituent, can be used to quantify inductive effects.Atoms or functional groups that are electronegative in comparison to hydrogen, such as the halogens, oxygen, nitrogen, and so on, may exhibit a negative inductive effect (-I), which is dependent on the order in which they are bonded to hydrogen. As a result, these atoms remove electron density from a compound’s single bond structure, which can aid in the stability of negative charge that may arise during reactions. The ionisation of acids is an example of a process in which -I groups can have a stabilising (enhancing) impact.
Take, for example, the cases of acetic acid, chloroacetic acid, and trichloroacetic acid. All three of these chemicals have the ability to ionise (loss of proton from the carboxyl OH). The sole distinction between these three structures is the extent to which the chloro group has been substitutedHydrogen has three isotopes: hydrogen, deuterium, and tritium. Hydrogen is a preferred propellant for nuclear-powered rockets and space transportation.
Hydrogen is also used to directly reduce iron ores to metallic iron and tungsten and molybdenum oxides to metals. A hydrogen (reducing) environment is used to pour specific castings, make magnesium, anneal metals, and cool huge electric motors. Inflating lighter-than-air vehicles like dirigibles and balloons used to be done using hydrogen, but now it’s done with helium. However, hydrogen-filled barrage balloons were deployed in WWII England. In the lab, liquid hydrogen is used to cool things down…
When applied to organic chemistry, the Hammett equation describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with only two parameters: a substituent constant and a reaction constant, which is defined as Louis Plack Hammett constructed and published this equation in 1937 as a follow-up to qualitative observations made in a 1935 article, which he had previously published. The fundamental concept is that, for any two reactions involving two aromatic reactions that differ only in the kind of substituent, the change in free energy of activation is proportional to the change in Gibbs free energy (and vice versa). This notion does not result from either elemental thermochemistry or chemical kinetics, and it was suggested by Hammett on the basis of intuition
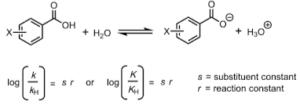
When it came to quantifying the influence of a certain substituent, Hammett needed a reference point. When he came up with his reference, he chose the ionisation of benzoic acid in water, and he formalised his concepts in his now-famous equation. It was determined that the reaction constant (r) for benzoic acid itself is equal to one, allowing for the calculation of a substituent constant (s) for each of 30 different substituents from log(k/kH) or log(K/KH), where k and K refer to substituted benzoic acids and kH and KH refer to the benzoic acid reference compound. In addition to its importance in substantiating notions about electronic substituent effects, this beautiful work also made significant contributions to mechanistic research. A time had passed since Ingold’s delineation of SN1 and SN2 mechanisms had made headlines10, and evidence for charged transition states had emerged from the Hammett equation as a result of the Ingold delineation. Recognizing Ingold’s view that it should be possible to forecast the effect of structure on reactivity4, Hammett had developed the first approach to support that belief.4 For additional reactions involving compounds that had been substituted with a group that had a s value, r could now be computed from a plot of log(k/kH) or log(K/KH) against s, as was previously possible. When he published his first report9, he included over 40 examples, and observed that k (or K) increases when a substituent is electron-withdrawing, and lowers when a substituent is electron-donating. The positive value of r, he hypothesised, corresponds to the formation of negative charge in the transition state (or product), whereas the negative value of r implies the growth of positive charge.
Conclusion
The relative inductive effect is caused by the sigma electron movement towards the more electronegative atom ” An arrow on the connection represents a permanent effect.” Alkyl group is less electron-withdrawing than hydrogen and is hence called electron-releasing. The +I effect indicates an electron-releasing character. Induction occurs when alkyl groups supply electrons. But this effect has been questioned. The Hammett equation is a widely used connection between structure and reactivity. This equation compares the reactivity of di- and polysubstituted benzene derivatives. Hydrogen has three isotopes: hydrogen, deuterium, and tritium. How do we tell them apart? They both have one proton (Z = 1) but have different numbers of neutrons. Deuterium has one neutron, tritium has two. The mass numbers of hydrogen isotopes are 1, 2, and 3. Their nuclei are 1H, 2H, and 3H. These isotopes’ atoms have one electron to balance one proton’s charge. Because chemistry is based on proton-electron interactions, the isotopes’ chemical characteristics are almost identical.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




