In organic chemistry resonance structures have the same atoms in the same arrangement, but the electrons can easily move around the molecule, spreading the charge around. Resonance structures can be used to depict this. The aromatic ring’s electrons are in resonance with the lone pair of electrons on the nitrogen atom in aniline, resulting in three potential resonance structures.
Isomerism refers to the potential of various molecules with the same atoms arranged differently. Two types of isomers are positional and functional. The three positional isomers of methyl pyridine are depicted in the diagram. Each of them is an aniline functional isotope. Each structure has the same number of each sort of atom, but they are arranged differently.
Resonance:
Resonant shape is another way to depict the Lewis point structure of a particular compound. The resonance form is the corresponding Lewis structure. These are used when there are multiple ways to place double bonds and lone pairs of electrons in an atom. Resonant structures occur when there are multiple ways to draw a Lewis scatter plot that satisfies the octet rule. Note that there are eight electrons in the outer shell, as the octet rule indicates that an atom acquires, loses, or shares an electron. Draw if the structure does not accurately represent the actual structure.
These are some of the basic principles of resonance theory. The first resonant structure is not real, it only shows the structures that can be connected. Resonant structures aren’t in sync with one another. The resonant structure is not an isomer. The isomers have different arrangements of atoms and electrons. Resonance morphology differs only in the arrangement of electrons. The resonance structure better represents the Lewis point structure because it clearly shows the intramolecular bonds. Not all resonant structures are the same, some are better than others. The better ones have the least formal charge, the formal negative charge is the atom with the highest electronegativity, and the bond is maximized within the structure. The more resonance morphology a molecule has, the more stable the molecule. They are drawn with double arrows between them to indicate that the actual structure is somewhere between the resonant structures. These structures used curved arrow notation to represent the movement of electrons from one resonance mode to the next.
Isomerism
Isomerism refers to the presence of molecules with the same number of the same type of atoms (and thus the same formula) but have distinct chemical and physical properties. The term isomer derives from the Greek isos plus meros, or “equal portions.” In layman’s terms, isomers are chemical molecules that have the same components but are not identical. To use a rough comparison, two bracelets composed of five red and five green beads each might be configured in a variety of isomeric configurations depending on the order of the colours.
Each bracelet would have the same components—five red and five green beads—but would be unique. Additionally, one could see combinations of those same beads in which pendant chains were joined in a variety of ways to a bracelet. Consider two bracelets in the same red-green colour scheme but with identical chains linked in opposite directions. These structures would also be comparable to isomers. A more nuanced parallel is that one’s hands are isomeric. Each hand has the same number of fingers, yet a right hand cannot be properly superimposed on a left hand; they are distinct.
Isomerism is also influenced by timing and energy. Molecules are mobile entities that undergo a variety of rotating motions that alter their forms, and these rotational motions consume energy. Thus, certain molecules may be identical on one timeline or set of energy circumstances but dissimilar, or isomeric, on another. Finally, an isomer must have the lowest possible energy; it must exist in an energy well.
Isomeric Effect
Compounds with the same molecular formula but differing structural formulae are known as isomers. Isomers of the same chemical might have various properties due to different structural arrangements. The isomeric effect refers to the change in the characteristics of compounds caused by isomers.
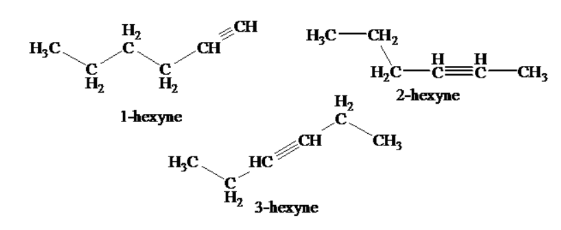
Consider the isomers of Hexyne, such as 1-Hexyne, 2-Hexyne, and 3-Hexyne.
Despite the fact that they are both isomers of Hexyne, 1-Hexyne is much more acidic because of the presence of terminal hydrogen, whereas 2 and 3 Hexyne do not. The isomeric effect refers to the changes in characteristics of hexyne isomers.
Lewis’s structure:
The Lewis structure symbolizes each atom and its position in the molecular structure. A line is drawn between the atoms that are bonded to each other (a pair of points can be used instead of a line). The excess electrons that form a lone pair of electrons are represented as a pair of dots and are placed next to the atom.
Conclusion:
The molecular formulas for isomerization are the same, but the molecular formulas are different. However, it resonates and delocalization of pi bonds occurs. Tautomers are separate compounds that can be separated and characterized in an appropriate manner, but the resonant structure is a fictitious structure of the same compound and cannot be separated.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




