In polyatomic ions or molecules, resonance structures are collections of Lewis structures that describe the delocalization of electrons in the atoms and molecules that make up the ion.
Because of the presence of partial charges and fractional bonds in a molecule/polyatomic ion, a single Lewis structure frequently fails to explain the bonding observed in the molecule/polyatomic ion. For chemical bonding in these situations, resonance structures are used to describe conclusions. According to the theory of valence bonding, resonance can be defined as a method of describing the bonding in specific molecules or ions by merging many contributory structures or forms, collectively known as canonical structures or resonance structures, into a hybrid resonance structure. Resonance can be defined as a method of describing the bonding in specific molecules or ions by merging many contributory structures or forms, collectively known as canonical structures or resonance structures (or hybrid structure).
It is sometimes necessary to distinguish between different resonance structures in order to determine which one(s) best describes the actual bonding in question. Formal charge can be used to predict which resonance structures will be preferred in a given situation.
Molecular Resonance Structures of the NO2-Ion
It is worth noting that the bond lengths of both nitrogen-oxygen bonds are the same in the nitrite ion. The Lewis dot structures of NO2– reveal a difference in the bond order of the two N-O bonds, which is particularly noticeable. As shown in the illustration below, the resonance hybrid of this polyatomic ion, which was created by combining its various resonance structures, can be used to explain the equal bond lengths.
Resonance Structures of Ion NO3-
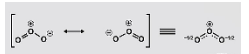
Nitrate ions are formed when nitrogen is the central atom in the ion. It is single-bonded to two oxygen atoms and doubly-bonded to one oxygen atom, according to the periodic table. Each of the oxygen atoms that is singly bonded to the nitrogen atom has a negative charge (in order to satisfy the octet configuration). The central nitrogen atom carries a positive charge, while the overall charge on the nitrate ion is a negative charge. These are the three possible resonance structures of NO3-, which are illustrated in the diagram below:
![]() Resonance Structures of O3
Resonance Structures of O3
Ozone (O3) molecules are composed of a central oxygen atom that is singly bonded to one oxygen atom and doubly bonded to another oxygen atom to form a molecule. Despite the fact that this molecule has no net charge, the Lewis structures of this molecule show that the central oxygen carries a charge of one and the singly bonded oxygen has a charge of one. These are the two resonance structures of the ozone molecule, which are illustrated in the diagram below.
With the oxygen atom at its core, ozone has positive charge; however, with the other oxygen atoms in the mixture, it has a negative charge {-(1/2)}.
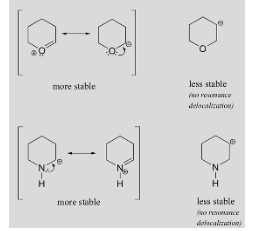
stability due to resonance
The resonance’s stability improves as follows:
- The quantity of covalent bonds
- The number of atoms having an octet of electrons is the number of atoms with an octet of electrons (except hydrogen which has a duplex)
- Separation of charges with opposing charges,
- Charge dispersion
- The stability of an atom is increased by a negative charge on a more electronegative atom and a positive charge on a more electropositive atom.
Resonance Structures of Nitrobenzene
Nitrobenzene contains an electron withdrawing group that is adjacent to the aromatic ring, which results in a lower electron density than in benzene. This is demonstrated by the resonance structures of nitrobenzene, which contain an electron withdrawing group that has a double bond that is adjacent to the phenyl ring of nitrobenzene.
As a result, the phenyl ring of nitrobenzene has a lower nucleophilicity than the benzene ring. These resonance structures indicate that the ortho and para positions are in fact positively positioned. The electrophile will not react at these positions, but will instead react at the meta position in an electrophilic aromatic substitution reaction. The electrophilic aromatic substitution product appears to be the meta substituted product if the phenyl [ring is in conjugation with a double bond, according to this theory.
Examples of resonance structures
1.Three atoms in an A=B-C configuration, with C being an atom with a p orbital:There are two major resonances that could occur. The difference between the two structures is the location of the double bond. Delocalization is a process that stabilises an anion, cation, or radical.
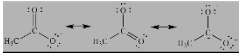
- Carboxylate Anion:
- Allylic carbocation
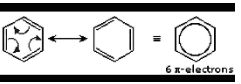
2.Conjugated double bonds:There are two distinct resonance structures in the benzene ring, which can be depicted by moving elections around in a circular fashion.
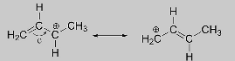
3.Cations adjacent to an atom with lone pair electrons:As a result of the greater electronegative nature of heteroatoms such as oxygen and nitrogen compared to carbon, you might expect them to act as electron withdrawing groups that destabilise carbocations.
4.Double bonds with one atom more electronegative than the other:The occurrence of multiple resonance structures in the molecule results in a charge separation in the molecule.
![]() Conclusion
Conclusion
Resonance structures are collections of Lewis structures that describe the delocalization of electrons in the atoms and molecules that make up the ion.Resonance can be defined as a method of describing the bonding in specific molecules or ions by merging many contributory structures or forms, collectively known as canonical structures or resonance structures.It is sometimes necessary to distinguish between different resonance structures in order to determine which one(s) best describes the actual bonding in question.
The electrophilic aromatic substitution product appears to be the meta substituted product if the phenyl [ring is in conjugation with a double bond. It is briefly discussed in this article how resonance structures of some molecules and polyatomic ions are formed.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





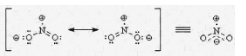
 Resonance Structures of O3
Resonance Structures of O3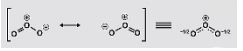
 Conclusion
Conclusion