Ammonia is one of the most widely manufactured chemicals in the United States. It’s one of the most frequent hydrides in the environment. Ammonia is generated in the atmosphere mostly as a result of bacterial degradation of nitrogenous compounds emitted by animals and plants. Azane is the IUPAC term for ammonia. Ammonia has the chemical formula NH3. Ammonia has a variety of properties.
Ammonia Natural Occurrence
Ammonia is a chemical that is created in small amounts in nature from nitrogenous animal and plant debris. Ammonia and ammonium salts can be found in minute amounts in rainwater, although ammonium chloride (sal ammoniac) and ammonium sulphate can be found in volcanic zones, and crystals of ammonium bicarbonate have been discovered in Patagonia guano. To neutralise excess acid, the kidneys produce ammonia. Ammonium salts are present in seawater and fertile soil.
Ammonia is also found on Mars, Jupiter, Saturn, Uranus, Neptune, and Pluto, among other places in the Solar System: on smaller, icy bodies like Pluto, ammonia can act as If the ammonia concentration is high enough, a mixture of water and ammonia can have a melting point as low as 173 K (100 °C; 148 °F), allowing such bodies to retain internal oceans and active volcanoes. Ammoniacal compounds are those that either contain or are similar to ammonia, allowing such bodies to retain internal oceans and active Ammoniacal substances are those that contain ammonia or are comparable to it.
Preparation of Azane/Ammonia
Due to the general breakdown of organic matter that is nitrogenous in origin, small amounts of azane/ammonia are present in soil and air. Ammonium salts react with caustic soda to produce ammonia on a small scale:
2NH4Cl + Ca(OH)2 → 2NH3 + 2H2O + CaCl2
Haber’s method is employed in large-scale manufacturing. Haber’s procedure includes the following step:
N2 (g) + 3H2 (g) ↔ 2NH3 (g)
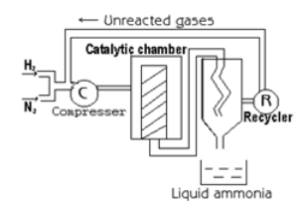
Nitrogen and hydrogen are used as raw materials for the reaction. The impurities for the gases are removed by a process called scrubbing. Following the scrubbing process, the gases are amalgamated and pushed via a compressor. After that, the mixture is crushed at 200 atm.
The compressed gases are then transferred via a converter, where they are heated to 450°C and pushed through a 200 atm pressure. Ammonia is generated when nitrogen combines with hydrogen, but only about 15% of the gas is produced. The mixture of ammonia, hydrogen and nitrogen is removed from the converter. It is cooled in the tank, where it liquefies and is collected.
Properties of Ammonia
The following are some of the characteristics of ammonia:
- Azane is a colourless gas with a pungent odour.
- At 198.4K and 239.7K, it boils.
- This gas dissolves quickly in water. Because OH- ions occur in the aqueous solution of NH3, it is a weak basis.
NH3 + H20 → NH4+ + OH–
- When ammonium combines with an acid, it forms ammonium salts.
ZnSO4 + 2NH4OH (g) → Zn(OH)2 + (NH4)2SO4 5.It has a density of 0.589 times that of air, making it lighter than air. It immediately liquefies due to the strong hydrogen interactions between molecules.
6.The symmetry of the crystal is cubic, with a Pearson symbol of cP16, a space group of P213 No.198, and a lattice constant of 0.5125 nm.
7.Liquid ammonia has significant ionising properties, as evidenced by its high ionisation number of 22.
8.Liquid ammonia has a very high standard enthalpy change of vaporisation (23.35 kJ/mol, vs. 40.65 kJ/mol for water, 8.19 kJ/mol for methane, and 14.6 kJ/mol for phosphine) and can thus be employed in uninsulated vessels in laboratories without further refrigeration. As a solvent, consider liquid ammonia.
9.In water, ammonia dissolves easily. It can be removed from an aqueous solution by boiling it. Ammonia is a basic aqueous solution. ‘.880 ammonia’ refers to the highest concentration of ammonia in water (a saturated solution) with a density of 0.880 g/cm3.
10.Ammonia does not burn easily or sustainably even in small fuel-to-air ratios of 15–25 percent air. When mixed with oxygen, it generates a weak yellowish-green flame. Nitrogen and hydrogen chloride is generated when chlorine interacts with ammonia; if chlorine is present in excess, the very explosive nitrogen trichloride (NCl3) is formed as well.
Conclusion
Ammonia is a colourless, odourless gas that has a strong odour. It is a stable binary hydride and is the simplest pnictogen hydride. It is a common nitrogenous waste among aquatic organisms, and it helps to meet the nutritional needs of terrestrial organisms by acting as a precursor.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






