The hydrazone is formed by condensation of the carbonyl molecule with hydrazine, and treatment with base causes the carbon to be reduced while the hydrazine is oxidised to gaseous nitrogen, yielding the equivalent alkane. The carbonyl group, C=O, is present in carbonyl compounds. Furthermore, acetaldehyde (CH3CHO) has been found to be common in the atmosphere, as well as a product of hydrocarbon combustion and photooxidation.
Carbonyl Compounds
Carbonyl compounds are chemical molecules that have a double bond between carbon and oxygen (>C=0). In organic chemistry, the most important functional group is >C=O.
Aldehydes are carbonyl compounds in which the carbonyl group is linked to a carbon and hydrogen.
Ketones are carbonyl compounds in which the carbonyl group is linked to carbon atoms.
Carboxylic acids and their derivatives are carbonyl compounds in which the carbonyl group is linked to oxygen (e.g. esters, anhydrides)
Amides are carbonyl chemicals that have carbon linked to nitrogen.
Aryl halides are carbonyl compounds that have carbon linked to halogen.
In a nutshell, there are two types of carbonyl compounds.
Identification Of Carbonyl Compounds
In a clean test tube, make a saturated solution of sodium bisulfite. 1 mL of the chemical substance to be examined is added. Shake vigorously and set aside for 15-20 minutes. If a white precipitate forms, the existence of the carbonyl group has been established.
Properties of Carbonyl Compounds
The following are some of the properties of carbonyl compounds
- These will be of a polar nature.
- They have a small amount of both positive and negative charge. As a result, these are referred to as polar molecules.
- Although these compounds are claimed to be insoluble in water, they do occasionally dissolve other polar molecules.
- Chemically reactive chemicals are what these are called. It signifies that they are in charge of a chemical reaction’s reactions.
Chemical Reactions of Carbonyl Compounds
The carbonyl group’s carbon atoms are known to be electrophilic because they attract electron-rich substances. Ions are electrophiles, but oxygen atoms are nucleophiles since they do not have a high density of electrons. They’re thought to be fond of nuclei like the bases. The reactions of carbonyl compounds are listed below
Carbonyl Reduction This reaction occurs when carbonyl groups are reduced by hydride reagents such as LiAlH4 and NaBH4 in the presence of baker’s yeast, or by catalytic hydrogenation.
Carbonyl alkylation Carbonyl alkylation is the process of alkylating carbonyl compounds with organometallic compounds such as Grignard reagents, organolithium reagents, acetylides, and so on.
Carbonyl Alpha-Substitution Reaction This type of substitution reaction includes an electrophile replacing a hydrogen atom.
Applications of Carbonyl Compounds
- Propanone, a carbonyl molecule that dissolves in water and other organic liquids, is utilised as a solvent .
- Formaldehyde is utilised in the production of polymers as well as in biological laboratories to preserve specimens .
- Butanol is used to keep the bread fresh by providing scent .
- In several chemical processes, acetaldehyde is utilised as a synthesiser .
Clemmensen Reduction
Simple ketones and aldehydes interacted with amalgamated zinc (Zn/Hg) in the presence of 40 percent aqueous hydrochloric acid and a hydrophobic solvent such as toluene to generate the equivalent alkanes after several hours under reflux conditions, according to Danish scientist Erik Christian Clemmensen. Since then, the Clemmensen reduction has been used to convert carbonyl groups to their corresponding methylene groups.
Because the Clemmensen reduction’s original severe conditions are incompatible with acid-sensitive substrates, numerous modifications have been attempted to raise the functional group tolerance and hence increase the reduction’s synthetic value. Yamamura and his colleagues devised a gentler method that involved employing organic solvents like tetrahydrofuran saturated with hydrogen halides like hydrogen chloride or bromide in the presence of activated zinc dust at ice-bath temperatures. Because some carbonyl compounds are poorly soluble in common solvents, a second solvent, such as acetic acid, ethanol, or dioxane, is used to improve solubility and speed up the reaction.
Biological features of many heterocyclic 1,3-dicarbonyl compounds with alkyl substituents at the electronegative “2” position are fascinating. Thomas Kappe and colleagues used a variation of the Clemmensen reduction to speed up the synthesis of many of these compounds.
Clemmensen Reduction Mechanism
Clemmensen reduction has a restriction in that the mechanism of this reaction is not well understood. There are two plausible mechanisms for the reduction. The carbanionic method is the first, in which zinc attacks the protonated carbonyl directly. The carbenoid mechanism, which is a radical process, is the second. The decrease takes place on the zinc metal’s surface. The carbanionic mechanism is demonstrated in this figure.
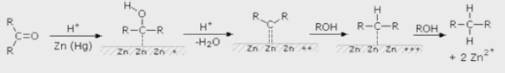
Conclusion
A carbonyl group is a functional group in organic chemistry that consists of a carbon atom double-bonded to an oxygen atom: C=O. It’s found in a variety of organic compounds, and it’s a component of many bigger functional groups. A carbonyl compound is a chemical compound that contains a carbonyl group. Clemmensen reduction is a chemical reaction in which ketones (or aldehydes) are converted to alkanes with the help of zinc amalgam and strong hydrochloric acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




