You are probably acquainted with the term ‘alcohol.’ It is a very common organic compound with a wide variety of practical applications on a large scale. Are you aware of the different ways of producing alcohol? This chapter will examine several industrial ways for manufacturing alcohols, including distillation.
Alcohols are the organic compounds with the functional group -OH attached. When this is attached to a benzenoid ring it becomes a phenolic alcohol.
Method of preparation of alcohol
There are several processes and procedures for manufacturing alcohol in businesses and laboratories. Let us examine them one by one to understand them better.
Preparation of alcohol from an alkyl halide
1) In the presence of water, alkyl halides are hydrolysed
As the name indicates, this is a nucleophilic substitution process. In the long term, this strategy is not especially effective. This is because it is dependent on external conditions and alkene byproduct may be obtained by elimination reaction.
R-X + H2O → R-OH + HX
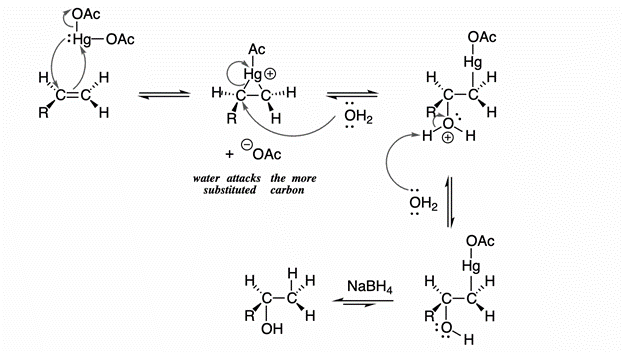
2) The mechanisms of oxymercuration and demarcation of alkanes
Mercury acetate is combined with alkenes in water and tetrahydrofuran to form alkyl mercury compounds. This is one of the most used methods for alcohol preparation. We must note that this mechanism always results in the markovnikov product, i.e the OH is attached at the position where the carbocation would be more stable. However this reaction doesnt involve any rearrangements. The mechanism is as follows:
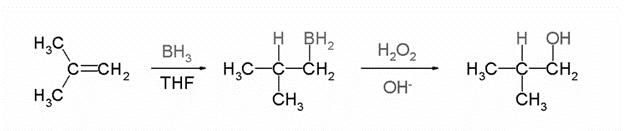
3)Hydroboration Oxidation
It is used to convert alkenes to anti-markovnikov alcohols. The -OH always attaches itself to the carbon containing more number of hydrogen bonded to it. The reagents used are B2H6 for hydroboration and H2O2 for oxidation. The mechanism is as follows:
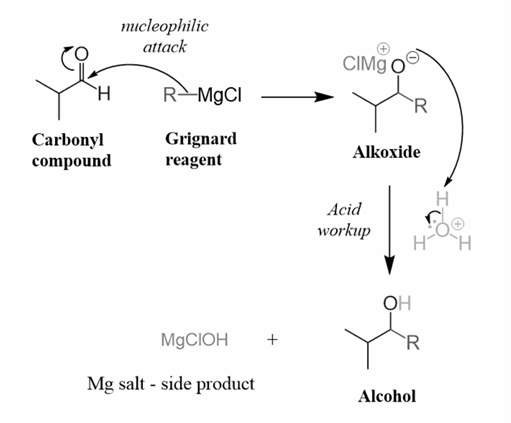
4) Grignard reagent
We can synthesise three distinct types of monohydric alcohols using Grignard reagents and carbonyl chemicals (primary, secondary, and tertiary alcohols). When RMgX is added to carbonyl compounds and hydrolysed, alcohols are formed. In its simplest form, the Grignard reagent is an organometallic compound. Let us take a deeper look at this answer since it is critical to comprehend.
We detect an explosive reaction between an alkyl halide solution in dry ethyl ether, (C2H5)O, and the alkyl chloride in the presence of metallic magnesium overturnings. The solution becomes hazy and evaporates. Magnesium metal gradually loses its characteristics. The Grignard reagent is the product of this reaction.
The Grignard reagent has the generic formula R MgX and is often called alkyl magnesium halide.
Additional information about Grignard reagent
The Grignard reagent is very reactive. It reacts with a wide variety of inorganic substances, including water, carbon dioxide, and oxygen, as well as the overwhelming majority of organic molecules found in nature. It is worth noting that an alkane is such a weak acid that it may be displaced by molecules that are generally considered very weak acids themselves or even chemicals that are not acids at all.
Preparation of alcohol via the Grignard synthesis
The kind of alcohol obtained from a Grignard synthesis depends on the carbonyl molecule used in the reaction: formaldehyde, HCHO, produces primary alcohols, while acetic acid produces secondary alcohols. On the other hand, aldehydes produce secondary alcohols, while ketones, R2CO, produce tertiary alcohols.
The reaction proceeds as follows:
5) By reduction of Carbonyl Compounds
The preparation of alcohol may also be formed by reducing aldehydes and ketones. Aldehydes can be converted into primary alcohols, while ketones can be converted into secondary. Catalytic hydrogenation of chemical reducing agents such as lithium aluminium hydride, LiAlH4 or NaBH4, may be used to perform this operation.
These reduction procedures are critical in synthesising some alcohols, which are less commonly available in the environment than their carbonyl equivalents. It is critical to remember that sodium borohydride (NaBH4) does not break carbon-carbon double bonds, even those conjugated with carbonyl groups.
6) The reduction process converts acids into alcohols
Lithium aluminium hydride (LiAlH4) is a rare reagent that converts an acid into alcohol. LiAlH4 is used to make lithium aluminium hydride. LiAlH4 is a common component in the laboratory for reducing acids and various other compounds. It is popular owing to the high yields it delivers. B2H6 ia another reagent that reduces acids to alcohols.
Conclusion
We have seen how alcohols are molecules that include one or more hydroxyl (-OH) groups directly connected to a carbon chain, as opposed to other compounds. Generally speaking, alcohols in the free-form are not found in nature; instead, they are found mostly in the essential or volatile oils extracted from the flowers, leaves, and stems of various plants.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




