In 1927, two physicists, Heitler and London, proposed the valence bond theory (VBT) to explain the creation of covalent bonds. Linus Pauling later improved on this notion by inventing the concept of hybridization. The VBT model is often referred to as the localized bond model. A covalent bond is created by the overlapping of the atomic orbitals of two combining atoms with unpaired electrons, according to valence bond theory.
Valence bond theory (VBT) is a quantum mechanical approach that uses localised quantum mechanics to describe the bonding in molecules. The Lewis interpretation of electron pairs forming bonds between atoms is mathematically justified by VBT. The valence bond theory postulates that all bonds are localized bonds established by the sharing of an electron between two atoms.
The following are the most important characteristics, postulates, and principles of valence bond theory:
- A covalent bond is created by the overlapping of half-filled atomic orbitals of combining atoms, which results in the formation of a molecule.
- Atomic orbitals that are subjected to overlap must be sufficiently close to one another and have the right orientation to do so.
- Only the valence orbitals of an atom overlap each other, resulting in the formation of a bond through the sharing of electrons.
- In overlapping orbitals, there is a pair of electrons with opposite spins that are present.
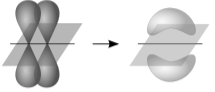
- The extent to which the overlapping occurs determines the strength of the bond formed. The stronger the covalent bond established as a result of the greater overlap of atomic orbitals.
- The total energy of the resultant molecule is less than the total energy of the individual atoms separated in time.
- It is important to note that the atoms that combine to form a molecule do not lose any of their individual identities when the molecule is formed.
Theory- A covalent link between two atoms is produced by the overlap of their half-filled valence atomic orbitals, each of which contains one unpaired electron. While a valence bond structure is similar to a Lewis structure, it is used in situations where a single Lewis structure cannot be written. Each of these VB structures is a Lewis structure in its own right. The primary focus of resonance theory is on this combination of valence bond configurations. According to the valence bond theory, a chemical bond is formed when the overlapping atomic orbitals of the involved atoms. Due to the overlapping, electrons are most likely to be in the bond region. Bonds are viewed as weakly connected orbitals in the Valence bond theory (small overlap). In general, valence bond theory is easier to apply to ground state molecules. During bond formation, the core orbitals and electrons stay basically unaltered.
Contrary to popular belief, overlapping atomic orbitals can be distinct. Sigma and pi are the two forms of overlapping orbitals. When the orbitals of two shared electrons collide head-to-head, sigma bonds form. Pi bonds form when two parallel orbitals collide. For instance, a sigma bond exists between two s-orbital electrons because two spheres are always coaxial. Single bonds have a single sigma bond, double bonds have a sigma bond and a pi bond, and triple bonds have a sigma bond and two pi bonds. However, the bonding atomic orbitals may be hybrids. Often, the bonding atomic orbitals exhibit characteristics of numerous different orbital types. Hybridisation is the process of obtaining an atomic orbital with the proper character for bonding.
The Valence Bond Theory’s Limitations
- Failure to account for the tetravalency of carbon.
- There is no information provided regarding the electrons’ energy.
- The concept is based on the assumption that electrons are concentrated in particular regions.
- It does not provide a quantitative assessment of coordination molecules’ thermodynamic or kinetic stabilities.
- There is no differentiation between weak and strong ligands.
- There is no reason for coordination compounds to be coloured.
- While it provides a subjectively pleasant pictorial representation of the complex, it does not provide quantitative insight into complex stability.
- It does not anticipate distortion in symmetrical compounds, but does in all copper (II) and titanium (III) complexes.
- It does not explain why the electrons must be arranged in opposition to Hund’s rule at times but not at others.
- In the absence of a source of energy, the theory occasionally requires electrons to be moved from a lower energy level to a higher energy level (Example 3d) (4p).
- Electron spin resonance reveals that the electron is not at the 4p level in Cu(II) complexes and that the complex is planar.
- It cannot account for why some complexes are more labile than others. Complexes that are labile are those in which one ligand is easily replaced by another. By contrast, inert complexes are those in which the ligand is gradually displaced.
Conclusion
The requirement of a maximum intersection is an important component of the Valence Bond theory, since it hints at the establishment of the strongest conceivable bonds. This idea is used to describe the formation of covalent bonds in a wide variety of compounds.
The Valence bond hypothesis is significant because it aids in the comprehension of the concept of molecule bonding. This article discussed the VBT postulates, their application, and contemporary VBT.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






