In their electron clouds, neutral nonpolar species exhibit spherically symmetric electron configurations. Their electron clouds can be altered in the presence of an electric field (Figure 1 ). The polarizability of an atom or molecule determines how easily it can be distorted. Because of the generated distortion of the electron cloud, the previously nonpolar molecule or atom gains a dipole moment. The following equation relates the induced dipole moment to the polarizability of the molecule or atom and the strength of the electric field:
uind =α E(1), where E is the electric field strength and is the polarizability of the atom or molecule in units of C m2V-1
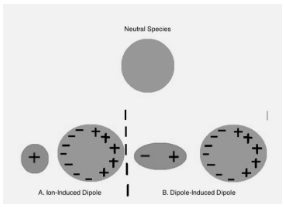
Figure 1: A neutral nonpolar species’ electron cloud is deformed to create a dipole moment by A.) an Ion and B.) a polar molecule.
Polarizability is generally related to the interaction of electrons with the nucleus. The number of electrons present in a molecule influences how tightly the nuclear charge can govern the total charge distribution. Because there is a significant interaction between the few electrons in the atoms’ orbitals and the positively charged nucleus, atoms with fewer electrons will have smaller, denser electron clouds. Atoms have less shielding because they have fewer electrons, which contributes to a stronger interaction between the outside electrons and the nucleus. Because the electrons in these tiny atoms are locked securely in place, they are not easily polarised by external electric fields. Large atoms with numerous electrons, such as negative ions with excess electrons, on the other hand, are easily polarised. These atoms have extraordinarily dispersed electron clouds and huge atomic radii, which limit the interaction between their exterior electrons and the nucleus.
Factors That Influence Polarisability
The following is the link between polarizability and the factors of electron density, atomic radius, and molecule orientation:
- The more electrons there are, the less control the nuclear charge has over charge dispersion, and consequently the greater the polarizability of the atom.
- The greater the electron distance from the nuclear charge, the less control the nuclear charge has over the charge distribution, and hence the greater the polarizability of the atom.
- Except for molecules that are tetrahedral, octahedral, or icosahedral, polarizibility can be affected by molecular orientation in relation to an electric field (labelled Orientation-dependent) (labeled Orientation-independent). This component is especially essential for unsaturated compounds with electron-dense sections, such as 2,4-hexadiene. When the electric field is applied parallel to the molecule rather than perpendicular to the molecule, the polarizability of these molecules is greatest.
Polarizability influences Dispersion Force
The weakest intermolecular force is the dispersion force. It is an attracting force caused by the presence of transient dipole moments in nonpolar molecules or species. When there are instantaneous deviations in the electron clouds of the nonpolar species, these temporary dipole moments appear. Surrounding molecules are impacted by these brief dipole moments, resulting in a chain reaction that produces further weak, dipole-induced dipole interactions. These dipole-induced dipole interactions result in attractive dispersion forces. When the temperature is low enough, dispersion forces cause nonpolar substances to condense into liquids and freeze into solids.
Polarizability has the following effects on dispersion forces:
- The dispersion forces become stronger as polarizability increases. As a result, molecules attract each other more strongly, and the melting and boiling temperatures of covalent compounds rise with increasing molecular mass.
- Polarazibility influences dispersion forces via the molecular form of the molecules affected. Elongated molecules have easily transported electrons, which increases their polarizability and hence strengthens the dispersion forces (Figure 2 ). Small, compact, symmetrical molecules, on the other hand, are less polarizable, resulting in smaller dispersion forces.
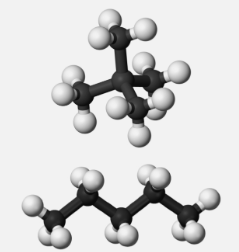
Pentane-3D-balls.png
Figure 2: A formalised paraphrase (top) Neopentane, an isomer of n-pentane, is an example of a less polarizable and more compact molecule. (bottom) n-Pentane is an example of a more readily polarised elongated molecule.
The following equation, which may be used to measure the interaction between two like nonpolar atoms or molecules, shows the link between polarizability and dispersion forces:
V=−3/4 *α2I/r6
where
r is the distance between the atoms or molecules,
I is the atom or molecule’s first ionisation energy,
α is the polarizability constant stated in units of m3.
The following equation connects this expression of to α′:
α′=α/4πϵ°
Conclusion
We conclude that in general, polarizability increases as electron volume increases. This happens in atoms because larger atoms have more loosely held electrons than smaller atoms with strongly bonded electrons. Polarizability decreases from left to right on rows of the periodic table.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



