Pinacol and pinacolone both contain alcohol functional groups, as implied by their names, whereas pinacol contains ketone functional groups. Pinacol is a compound that contains two hydroxyl groups, each of which is attached to a carbon atom on either side of the compound. Pinacol is officially known as 2,3-Dimethylbutane-2,3-diol by the International Union of Pure and Applied Chemistry.
Pinacolone is an organic compound that contains functional groups associated with the ketone ring. It is a clear, colourless liquid with a peppermint or camphor odour that lingers after application. Pinacolone is officially known as 3,3-Dimethyl-2-butanone by the International Union of Pure and Applied Chemistry.
Pinacol Pinacolone Rearrangement Reaction
The reaction in which an alcohol with two adjacent alcohol groups (pinacol) is converted to a ketone (pinacolone) under the influence of an acid (catalyst) is known as the pinacol pinacolone rearrangement reaction. Fittig’s observation of the rearrangement reaction in the year 1859 is credited with triggering the reaction. The 2, 3-dimethyl-2, 3-butanediol reacts with an acid, causing the rearrangement of the ring system. The precise composition of the product was not known at the time. A carbon skeleton rearrangement was discovered by Burlerov, who identified the product’s structure as 3,3-dimethyl-2-butanone and labelled it as the result of such a rearrangement.
During the course of this organic reaction, protonation of one of the –OH groups takes place, resulting in the formation of a carbocation. If the –OH groups are not identical (i.e., if the pinacol is asymmetrical), then the one that produces a more stable carbocation participates in the reaction, and the others are excluded. An alkyl group from the adjacent carbon migrates to the carbocation centre as a result of this process.
This organic reaction, known as pinacol rearrangement, is characterised by the dehydration of alcohol, which results in the formation of ketones. Whenever concentrated sulfuric acid, heat, and boiling chips combine to catalyse the pinacol rearrangement among 1,2 diols such as glycols, a reaction such as this one is possible. The name of a reaction is always derived from the name of the reactant involved. Pinacol is an alcohol that undergoes rearrangement to become a ketone in the body. This reaction is similar to the reaction pathway of SN1, in that it results in the creation of a carbonyl group from a diol as the end product. When pinacolone is suspected, the 2,4-DNP test is used to confirm the suspicion. Using this test, you can determine whether or not there are any aldehydes or ketones present in organic molecules. In this scenario, the test is useful in determining whether the product is a ketone, such as pinacolone, or not. After a few drops of the reagent are added to the product, an organic precipitate is formed, which confirms the presence of the carbonyl group in the product.
Mechanism of Pinacolone Rearrangement Reaction
Following are the four phases that take place during the Pinacol Pinacolone rearrangement reaction mechanism:
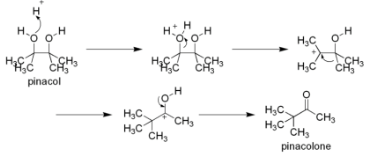
1. Protonation of a hydroxyl group is the first step.
Pinacol is bonded to the hydroxyl group in this stage by a proton or hydrogen ion that has been released from the acid (H2SO4 breaks down into H+ and HSO4–ions).
2. Depletion of the water molecule
As a result of this step, water molecules are released from the chemical, causing carbocation.
3. Methyl migration is the third step.
It was during this step that the methyl group was transferred from one carbon atom to another carbon atom. It attaches itself to a positively charged carbon atom in the carbocation molecule.
4. Deprotonation is the fourth step.
Because the proton that was attached to pinacol in the first step was generated by an acid catalyst during the reaction, it is detached in this step. It is the final product pinacolone that is formed when a proton is detached from a carbocation.
Applications of Pinacolone (Product of Pinacol Rearrangement)
C6H12O is the chemical formula for pinacolone, which is a colourless liquid. It has a wide range of applications, including the production of fungicides, herbicides, pesticides, and pharmaceutical products. The following are some examples of how pinacolone is used:
- When analysing vibunazole, pinacolone is used as a tool.
- In the synthesis of pinacidil and naminidil, it is a necessary component.
- Fungicides, herbicides, and other pesticides are all made with it.
- Tridimefon, paclobutrazol, uniconazole, and metribuzin are all manufactured using this chemical.
- In the pharmaceutical industry, it is extremely useful.
Conclusion
When 1,2-diols react with each other, this is known as the pinacol rearrangement. It takes place under the influence of strong acids, which can include mineral acids such as sulfuric acid, as well as other chemicals. In addition, the use of Lewis acids can bring about this effect. The loss of one of the hydroxyl groups, the conversion of the other hydroxyl group into a carbonyl group, and the shift of an alkyl group are all part of the overall reaction.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






