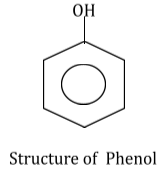
Phenol is an organic compound and its structural formula is C6H5OH. It consists of one phenyl group and –OH (hydroxyl) group attached to it.
Phenol is a liquid with a higher boiling point than alcohol because they have stronger intermolecular hydrogen bonding which is soluble in water. Phenol is a strong acid. Introducing electron-withdrawing groups such as NO2or CN, on the ring increase the acidity of phenol, and introducing electron-donating groups such as NH2, R or OR decrease the acidity of phenols. It is due to the effective delocalization of negative charge in phenoxide ions.
Properties of Phenol
Phenol is colorless, crystalline solid, and also deliquescent. The word deliquescent means the process in which any substance absorbs moisture and changes into a liquid state. It has a melting point of 41 degrees Celsius and a boiling point of approximately 182 degrees Celsius. It is soluble in water forming a pink solution at room temperature. It is completely soluble at 65.9 degrees Celsius. It is also poisonous and is used as a disinfectant in hospitals and bathrooms.
Boiling Point of Phenol: Generally, Phenol has a high boiling point compared to other hydrocarbons which have equal molecular mass. And, it is due to the presence of intermolecular hydrogen bonding between the hydroxyl group and phenol molecules. Normally, the boiling point of phenol is 181.7 degrees Celsius.
Acidity of Phenol: Phenol is highly acidic in nature. Phenol can react with metals like Sodium, Potassium, etc and they form phenoxide ions. pKa value of phenol is 9.95 in water and 2.91 in acetonitrile.
Solubility of Phenol: The solubility of phenol generally decreases with an increase in aryl group size. Hydrogen bonds between water and phenol molecules make phenol to be soluble in water.
These are some common properties of phenol. Now, we will discuss the preparation methods of phenol.
Hydrogen bonds in Phenol-
Due to strong intermolecular hydrogen bonding, phenols have higher boiling point than the respective hydrocarbon and aryl halides. As they can form hydrogen bonds with water, phenols are soluble in water. As we have seen, phenols are acidic in nature and are stronger acidic compared to alcohols. This is because sp2 hybridized carbon of phenols to which –OH is attached is electronegative. It results in a decrease in electron density on oxygen. This, however, increases the polarity of the O-H bond and would result in an increase in ionization of phenols than that of alcohols.
Some Applications of Phenol
As an injection
To treat muscle spasms, we use phenol as an injection. During severe cases of stiffness and also ingrown toenails, phenol is used for the treatment. Phenol helps us to relax and limit the signals sent to the brain.
As a Preservative
Phenol is used as the preservative of vaccination doses for polio, typhoid, pneumonia, etc.
As a chemical peel
Phenol-derived compounds can be used in chemical peels. They repair damaged skin from the surface. These compounds are not very safe for the skin, they are highly mild when used on the skin. For example, phenol of trichloroacetic acid.
As Cosmetic and Food Preservation
Phenol is also used as a food and cosmetics preservative. Its safety is approved by the FDA. But, due to the toxicity of phenol-derived BHT (butylhydroxytoluene), it is banned. Hence, these are some applications of phenol. The areas and fields where phenol is used in daily lives.
Conclusion
In this article, we discussed phenol. Also, we read about phenol and its basic properties and its applications. We came to know that phenol is also used in daily life. We have seen multiple uses of phenol. A few are as mentioned- Phenol is used in cosmetics and food preservation, used in chemical peels, used as a preservative, and also used as an injection. In much detail, we have even talked about the physical properties of phenol.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out