Introduction
The physical properties of Aldehydes and ketones are very similar with the presence of the carbonyl group in both aldehydes and ketones. However, the carbonyl group has a polar character, implying that the shared pair of electrons between carbon and oxygen is more inclined towards oxygen than carbon. Since oxygen, being more electronegative than carbon, attracts the shared pair of electrons and causes a slight negative charge to accumulate over itself, whilst the carbonyl carbon atom gets a small positive charge over itself, this is the result.
Aldehydes: physical properties of aldehydes
Aldehydes are compounds in which hydrogen and carbon have been connected to carbonyl groups. Aldehydes are organic compounds and types of carbonyl groups surrounded by hydrogen and alkyl groups composed of the carbonyl group (R). The carbonyl group in aldehyde is composed of one alkyl group and one hydrogen group on the other end. The aldehyde in its condensed form is denoted by the suffix -CHO. Ar and R, respectively, represent the aryl and alkyl components.
Physical Properties of Aldehydes
Because both ketones and aldehydes include the carbonyl functional group, they are often used interchangeably in chemical processes. However, they have significant differences in the most critical processes, such as oxidation. Ketones are resistant to oxidation, but aldehydes are easily converted to carboxylic acids by the action of oxygen. Aldehydes are a class of chemical compounds that are easily oxidised by the environment. The ease with which they oxidise aids in their identification.
- Physical State
In its gaseous state at ambient temperature, formaldehyde, while acetaldehyde is a volatile liquid, are two different chemicals. Therefore, C11 and below are colourless liquids, while C12 and higher are solids. All other aldehydes and ketones are colourless liquids.
- Tautomerism
An aldehyde’s oxygen atom can migrate to the carbonyl group’s hydrogen atom if it has at least one hydrogen atom on the carbon atom next to it, termed the alpha () carbon. Afterwards, the double bond moves toward the carbon. Tautomers are two isomeric forms of carbonyl compounds having a -hydrogen. There are two distinct forms of ketosis, with the hydrogen connected to the -carbon and the carbonyl oxygen linked by the migration of the double bond.
- Boiling Point
Because they are non-polar chemicals, ketone and aldehyde boiling points are greater than those of the other non-polar compounds. Although the boiling temperatures of ketones and aldehydes are lower than those of the equivalent carboxylic acids and alcohols, this is due to the fact that they do not form hydrogen bonds with one another.
- Dipole Moment
A double bond exists between carbon and oxygen in the carbonyl group of aldehydes and ketones. In addition, because oxygen has a higher electronegative charge than carbon, it draws the shared pair of electrons, making the carbonyl group more reactive and polar. As a result, aldehydes and ketones have strong dipole moments.
- Solubility
Hydrogen bonding makes it possible for the lower members of the ketones and aldehydes to be soluble in water, and this is especially true for those with up to four carbons in their structure. However, their higher members do not dissolve in water due to the high concentration of hydrocarbons in the solution, which prevents the formation of H-bonds with the water molecules in the solution. In addition, there are dipole-dipole interactions and dispersive forces between the water molecules and the aldehydes and ketones.
Chemical Properties of Aldehydes
- Nucleophilic addition
Nucleophilic addition reactions are a type of chemical reaction in which two nucleophiles combine to form a nucleophile.
The following mechanisms characterise the nucleophilic addition reactions:
In the polar carbonyl group, a nucleophile contacts the electrophilic carbon atom from a direction that is essentially perpendicular to the plane of the sp2 hybridised orbitals of the polar carbonyl group.
A tetrahedral alkoxide intermediate is formed due to the hybridisation of carbon, which shifts from sp2 to sp3 in this reaction. A proton is captured by this intermediate and transferred to the reaction medium, yielding the electrically neutral product. You get a net outcome when you put Nu– and H+ together across the carbon-oxygen double bond.
- Reactivity
Reactivity includes steric and electronic differences, making aldehydes more reactive than ketones in nucleophilic addition processes. In addition, because ketones have two relatively large substituents, the approach of a nucleophile to carbonyl carbon is more difficult in ketones than in aldehydes, which contain only one of these substituents.
- Reaction with Hydrogen Cyanide
Aldehydes and ketones react with hydrogen cyanide (HCN) to produce cyanohydrins, a type of cyanide compound. In the presence of pure HCN, this reaction takes place very slowly. Therefore, a base catalyses it, and the cyanide ion (CN–), a stronger nucleophile than carbonyl compounds, quickly reacts with them to produce the appropriate cyanohydrin. A class of synthetic intermediates known as cyanohydrins exists.
- Addition of sodium hydrogen sulphite
Sodium hydrogen sulfite reacts with aldehydes and ketones to create the compounds known as additional products.
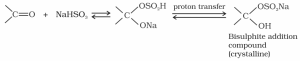
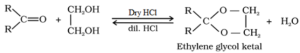
- Addition of Alcohol
When combined with monohydric alcohol in the presence of dry HCl, Aldehydes generate hemiacetal, which, when combined with one additional molecule of alcohol, transforms into acetal.
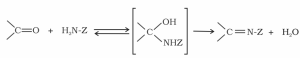
- Addition of ammonia and its derivatives
Adding ammonia and its derivatives to aldehydes and ketones: Nucleophiles such as ammonia and its derivatives H2N-Z react with the carbonyl group of aldehydes and ketones to form a bond.
Z can be any of the following: alkyl, aryl, OH, NH2, C6H5NH, NHCONH2, or any other combination.
- Reduction
When aldehydes and ketones react with sodium borohydride (NaBH4) or lithium aluminium hydroxide, they form primary and secondary alcohols, respectively (LiAlH4).
Conclusion: aldehydes and ketones physical properties
The carbonyl group (>C=O) is present in aldehyde. The nature of the carbonyl group I has a significant impact on the physical properties of aldehydes. This article provided an overview of the physical properties of aldehyde and ketone with a focus on the solubility and boiling points of these organic substances. It was also revealed that the boiling point of these compounds is higher than that of their hydrocarbon equivalents. We also learnt why alcohols and carboxylic acids have greater boiling temperatures than their comparable aldehyde and ketone acid counterparts, which we discussed previously.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




