Introduction:
Peroxydisulfuric acid is a peroxyacid oxidant with a lot of potency. Marshall’s acid is another name for peroxydisulfuric acid, which was named after its inventor, Prof. Hugh Marshall. It’s a sulphuric and peroxydisulfuric acid anhydride produced by oxidizing oleum with ozone or hydrogen peroxide.
In an electrolysis reactor, an aqueous solution containing sulfate ions can be electrolyzed to produce peroxydisulfuric acid.Other names for peroxydisulfuric acid include sulfo oxy hydrogen sulfate and persulfuric acid.
Peroxydisulfuric acid formula:
H2S2O8 is the chemical formula for peroxydisulfuric acid, an inorganic molecule. It can be written as HO3SOOSO3H in structural terms. It has a peroxide group and sulphur in its +6 oxidation state.
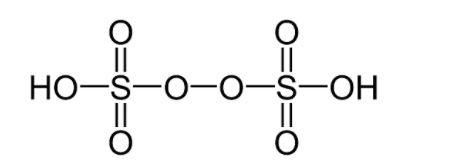
Peroxydisulfuric acid preparation:
Concentrated sulfuric acid and sodium persulfate are combined to make peroxydisulfuric acid. Chlorosulfonic acid and a hydrogen peroxide procedure can alternatively be used to make this acid. The solid form is far more difficult to make and is utilized as a solution more commonly. The following is the chemical equation for preparing peroxydisulfuric acid:
2CISO3H + H2O2 → H2S2O8 + 2HCl
Physical properties:
Peroxydisulfuric acid has the following physical properties:
- The solid peroxydisulfuric acid is colorless and odorless.
- It is water soluble.
- It has 8 hydrogen bond acceptors and 1 covalently bonded unit.
- It has a ten-atom heavy atom count.
Chemical properties:
Peroxydisulfuric acid has the following chemical properties:
- Sulfuric acid and hydrogen peroxide are produced when peroxydisulfuric acid dissolves in water. The chemical equation for this is as follows:
H2S2O8 + 2H2O → H2O2 + 2H2SO4
- Silver oxide, nitric acid, and sulphuric acid are formed when peroxydisulfuric acid reacts with silver nitrate in an aqueous media. The chemical equation for this is as follows:
H2S2O8 + 2AgNO3 + 2H2O → Ag2O2 + 2H2SO4+ 2HNO3
Uses:
Peroxydisulfuric acid is used for the following purposes:
- Large-scale sulphuric acid synthesis was made possible by the use of peroxydisulfuric acid and its salts as a source of hydrogen peroxide.
- In photography, peroxydisulfuric acid can be employed as a hypo eliminator.
- It can also be employed as a strong oxidant, with the amount of oxidizing agent used varying based on the reaction rate desired.
What kind of acid can burn your skin?
One of the most serious chemical injuries to tissues is a sulfuric acid burn. Consider its characteristics, first aid, treatment options, and prevention techniques.Chemical burns are unique in that once the reagent enters the skin, a crust forms that looks almost like healthy tissues. The scab is visible on the surface, and the skin turns white, then brown. A purple crust forms when the wound heals. Acid can cause vision loss if it gets into your eyes. A laryngeal burn happens if you inhale its vapours. Hemorrhagic pneumonia and mortality are caused by high reagent concentrations.
Conclusion:
Peroxydisulfuric Acid is described as a colourless solid and also one of the most powerful peroxy acid oxidants that are available currently. This Acid is an inorganic compound having the chemical formula H2S2O8. It can be generated either from ammonium persulfate or potassium in an acidic solution. It is given as an anhydride of peroxydisulfuric and sulphuric acid and can be prepared by the oxidation of oleum either with ozone or hydrogen peroxide. This is also known as Marshall’s acid; peroxydisulfuric acid is produced by electrolyzing an aqueous solution that contains sulfate ions in an electrolysis reactor should be produced by process of electrolyzing.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




