Molecular oxygen, or simply oxygen, is the regular oxygen we breathe and is present all over the atmosphere. This molecular oxygen is split apart by the sun’s rays into two single oxygen atoms. These atoms then recombine with an oxygen molecule to form Ozone(O₃).
Ozone is a pale blue-coloured poisonous gas with a typical pungent smell, while liquid Ozone is deep blue and strongly magnetic. It is much less stable than oxygen and is considered a pollutant, having adverse effects for humans and other creatures if present too close to the ground.
The vast majority of Ozone is accumulated in the stratosphere (a layer of atmosphere between 10 to 50 kilometers above Earth’s surface). Ozone adds up to roughly 0.00006% of the atmosphere, and the highest concentrations are present at 32 km above the surface, in an area known as the “ozone layer.”![]()
Formation of Ozone
When an oxygen molecule receives a photon, it dissociates into monoatomic reactive atoms. These atoms combine with an oxygen molecule to form ozone, O₃. O₂ + hν → [O] + [O] O₂ + [O] → O₃Structure of Ozone
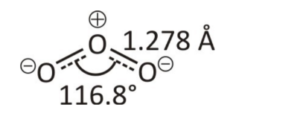
Ozone has three oxygen atoms, but it cannot form a triangular structure due to steric hindrance. Each oxygen atom in the ozone molecule forms a single bond, and the remaining negative charge spreads throughout the molecule.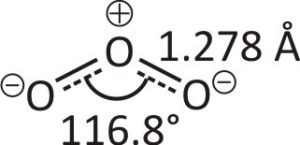
Resonance of Ozone Molecule
Resonance describes delocalised electrons bonding with specific molecules or polyatomic ions where a single Lewis-dot structure cannot explain the bonding. Combining these possible resonance structures represents the overall delocalisation of electrons within the molecule and is called the resonance hybrid. Two main resonance structures in ozone contribute similarly to the overall hybrid structure of the molecule. These structures represent the 18 required valence electrons-6 out of 3 bonds and 12 electrons as lone pairs placed on the oxygen atoms.Ozone Layer
The ozone layer is a significant part of Earth’s stratosphere that absorbs most of the sun’s ultraviolet radiation(a form of non-ionizing radiation with wavelengths varying from 10 nm to 400 nm). Although lower than other gasses in the stratosphere, it contains a higher ozone concentration (O₃) than other parts of the atmosphere. This layer contains less than ten parts per million(10ppm) of ozone, while the average ozone concentration in the entirety of Earth’s atmosphere is about 0.3 parts per million(0.3ppm). The lower layer of the stratosphere mainly houses the ozone layer, although the thickness varies seasonally and geographically.Benefits of Ozone Layer
- The sun and other artificial sources like the tanning beds emit UV radiation(UV-A, UV-B, and UV-C). This radiation is carcinogenic and causes skin cancer and cataract in humans. The Ozone Layer absorbs this radiation to a great extent(it mainly absorbs the UV-B), limiting the harmful radiation which reaches the Earth’s surface, thus protecting humans
- It also prevents radiation damage to plants as UV radiation can directly affect plant growth, influencing their physiological and developmental progress
- It protects synthetic polymers and some naturally occurring biopolymers as they undergo breakdown with exposure to UV radiation, reducing their commercial usability
- An increase in this UV-B radiation could influence the terrestrial and aquatic biogeochemical cycles, thus altering the sources and the sinks of greenhouse gases like carbon dioxide and carbon monoxide. These changes could directly contribute to the amplification of the atmospheric concentration of those gases
Depletion of Ozone Layer
Ozone layer depletion refers to the gradual thinning of the Earth’s ozone layer in the stratosphere, which is primarily caused due to the release of chemical compounds containing gaseous bromine(Br) or chlorine(Cl) from industries or other human activities. The chlorine and bromine atoms contact the ozone molecules and destroy them. Research says that one chlorine atom is capable enough of destroying 1,00,000 ozone molecules.Ozone Depleting Substances(ODS)
Some compounds release chlorine and bromine on exposure to high ultraviolet light, contributing to ozone layer depletion. These compounds are called Ozone Depleting Substances (ODS). The ODS which contain chlorine include chlorofluorocarbon(CFC), carbon tetrachloride, hydrochlorofluorocarbons, and methyl chloroform. The ODS, which are halides of bromine, include methyl bromide, halons, and hydro bromofluorocarbons. The most abundant Ozone Depleting Substances are chlorofluorocarbons(CFC). Only when the chlorine atom reacts with some other molecule does it not react with ozone.Montreal Protocol (1987)
The Montreal Protocol was proposed in the year 1987 to stop the use, production, and import of ODS to minimize their concentration in the atmosphere, thereby protecting the ozone layer.Challenges Faced While Protecting the Ozone Layer
Scientists warned us forty years ago that a hole in the ozone layer encircling the Earth might have significant consequences for human health and the environment. This issue has been resolved due to a global agreement to prohibit the use of ozone-depleting substances that harm the ozone layer. However, scientists are increasingly concerned that the compounds used to replace these ozone-depleting chemicals are trapping heat inside the planet, aggravating the greenhouse effect(when gasses in the Earth’s atmosphere trap the sun’s heat, making Earth warmer).Tropospheric Ozone
The troposphere is the Earth’s atmosphere’s lowest layer, containing the majority of the atmosphere’s mass (about 75-80 per cent). The interaction of sunlight, particularly ultraviolet light, with hydrocarbons and nitrogen oxides released by automotive tailpipes and smokestacks produces tropospheric ozone. Human activities on the Earth’s surface, close to the troposphere, result in ozone concentrations several times higher than the natural background level. It is ‘bad’ to have too much of this ground-level ozone because it is harmful to one’s health and damages vegetation.Harmful Health Effects of Ozone
- Throat irritation, coughing, and an uncomfortable sensation in the chest are all symptoms of respiratory system irritation. People with existing respiratory conditions, like asthma, chronic obstructive pulmonary disease (COPD), lung cancer, and those who spend a significant amount of time outdoors are particularly vulnerable to the effects of ozone
- Reduced lung function makes it more challenging to take deep and vigorous breaths, making it more challenging to exercise. People’s breathing may become more rapid and shallow than usual, and their ability to engage in vigorous activities may be limited as a result. Wheezing and shortness of breath are caused by ozone because it causes the muscles in the airways to constrict, causing air to become trapped in the alveoli
- Asthma flare-ups are becoming more common. With a hike in ozone level, more people with asthma experience attacks that necessitate a doctor’s consultation or medication administration. One of the primary reasons for this is an increase in ozone concentration. Ozone makes people more sensitive to allergens, which triggers asthma attacks in susceptible individuals
- Respiratory infections are more likely to occur due to increased ozone concentration
- When the concentration of ozone increases, the lung lining is inflamed and damaged. The damaged cells are shed and replaced within a few days, like the skin peels after a sunburn. Suppose this type of inflammation repeatedly occurs over a long period (months, years, or a lifetime); studies suggest that lung tissue may become permanently scarred, resulting in permanent loss of lung function as well as decreased quality of life
- More recent research suggests that ozone can also have harmful effects on the body through the inflammatory pathway, resulting in heart disease, type 2 diabetes, and other metabolic disorders
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out