Metallurgy is the process wherein metals are extracted from ores. This process is made much easier with the help of thermodynamic principles. Thermodynamics is the branch of scientific study that deals with the relationship of thermal energy, that is, heat, with other forms of energy. It is the study of the energy that is transferred during the chemical and physical changes – it lets us predict and measure the changes.
Use of thermodynamics in metallurgy
The main concept of thermodynamics that comes up with metallurgy is the Gibbs Free Energy model. In the thermodynamic study, the concept of Gibbs Free Energy determines whether a particular process will occur spontaneously or not. It is useful to evaluate the ease of reduction of metal oxides and sulphides. The symbol for it is Δ G. So, if we consider the value for the ΔG to be negative, then the reaction that occurs will be negative. There are two types of equations to arrive at this Δ G, which is stated as follows –
ΔG = ΔH – T Δ S
In this equation, Δ H is taken as the change in the enthalpy. So in this equation, a positive value will automatically come up with an endothermic reaction, whereas with a negative value, it will automatically be an exothermic reaction. Thus, if the reaction is an exothermic one, it will hence make the Δ G negative. However, this will take a sharp change whenever the subject matter changes. Thus, if we look at a different equation that relates the Gibbs Free Energy to the equilibrium constant, it will look as follows –
Δ G ° = – RT ln K eq
The Keq in this equation will be the equilibrium constant. It is calculated if we divide the active mass of the given products by the active mass of the given reactants. The R in this equation is the universal gas component. Thus, to reach the negative value of the desirable ΔG, the equilibrium’s value must always be kept on the positive side.
The Ellingham Diagram
With the help of the Ellingham Diagram, the relation between the stability and temperature of a compound can be obtained. This is the graphical presentation of the model of Gibbs Energy Flow. In the case of metallurgy, the Ellingham Diagram is used to plot the equations of the reduction process as this helps the user reach the most suitable reducing agent when the oxides are reduced to give out pure metals. It helps to determine the feasibility of the thermal reduction of ore. We have discussed the important properties of the Ellingham Diagram as follows –
- In this case, the Δ G has been plotted to have a relation to the temperature. Thus, the slope of the curve will represent the entropy, whereas the intercept will represent the enthalpy.
- The Δ H will not be affected by the temperature.
- The Δ S, which is the entropy, will not be affected by the temperature either. Keep in mind that there is a condition to it – the phase change should not happen.
- The temperature will need to be plotted on the Y axis, whereas the Δ G shall be plotted on the X-axis.
- Metals that come with a curve at the bottom of the diagram will reduce the metals located at the top.
Thus, the reaction of the metal with the air can be represented as follows –
M (s) + O2 (g) → MO (s)
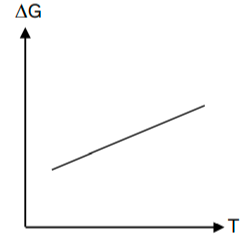
What are the exceptions to the Ellingham Diagram?
In the case that the entropy is negative, the slope shall not go upwards. There are a few instances where this can occur –
C(s) + O2 (g) → CO2 (g) – in this case, the entropy of the solids are negligible. Thus, a single molecule of gas will result in a single molecule of gas. So, there is almost no entropy at all. There will be no slope – the line will be completely horizontal.
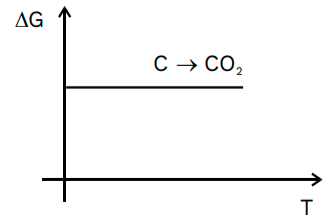
Applications of the Ellingham Diagram
There are numerous applications of the Ellingham Diagram, which are stated as follows –
- The Alumino Thermic Process: when it comes to the Ellingham Diagram, the graph usually lies lower than the other metals, including iron. Thus, it means that aluminium can be used as a reducing agent for oxides of those metals that fall higher up than it on the graph.
- Extracting iron: iron is extracted in a blast furnace. In a blast furnace, the ore is combined with limestone and coke. However, the reduction of the iron oxides occurs at a different temperature. The furnace’s lower part is set to a higher temperature than its top part.
Conclusion
Metals are found in ores, and they are generally in the form of combination with other types of elements or metals. Thus, as a result, once the metal ore has been extracted from the ground, they need to be converted into its pure metal form. The main concept of thermodynamics that comes up with metallurgy is the Gibbs Free Energy model. In the thermodynamic study, the concept of Gibbs Free Energy determines whether a particular process will occur spontaneously or not. It is useful to evaluate the ease of reduction of metal oxides and sulphides.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






