Methods of Preparation of Phenols:
The phenol has a chemical formula of C6H5OH. White crystalline powder is very flammable. A covalent bond is formed between the phenyl and the hydroxyl groups in this molecule.
It was once necessary to mine coal tar to obtain phenol, but it is now produced in huge quantities from petroleum (approximately 7 billion kilograms per year). It’s a vital part of the manufacturing process. Most of the time, it’s used to produce plastics and other similar items.
Preparation of phenol:
Several chemicals are utilised to make phenols, including diazonium salts, sulfuric benzoic acid, haloarenes, and cumene. Carbolic acids are another name for these acids. Weak acids are formed when the hydroxyl group loses one hydrogen ion and produces phenoxide ions. Derivatives of benzene can be found in a variety of items.
In labs, it’s used to make phenols. The steps involved are listed below:
1. Haloarenes are used to make phenols.
Chlorobenzene is a haloarene formed when the benzene ring is replaced with a chlorine atom. At 623K & 320 atm, sodium phenoxide is obtained when chlorobenzene reacts with sodium hydroxide. On acidification, heating sodium phenoxide produces phenols. 300 atm , 623K HCl
+ ——————> ————–>
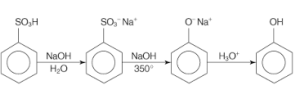
2. Phenol Preparation from Benzene Sulphonic Acid:
Sulphonic benzene acid is made by reacting benzene with oleum. It is then fused with sodium hydroxide (molten state) at a high temperature, resulting in sodium phenoxide. Lastly, when sodium phenoxide is acidified, it produces phenols.
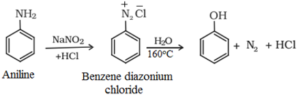
3. Phenol Preparation from Diazonium Salts:
Diazonium salts are formed when a primary aromatic amine is treated with nitrous () acid at 273-278 K.
These diazonium salts finally hydrolyse to phenols when heated with water. Dilute acid can be used to cure diazonium salts, and phenols can be obtained.
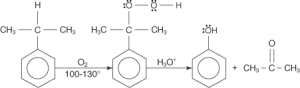
4. Phenol Preparation Using Cumene:
When cumene (isopropylbenzene) is oxidised in the presence of air, cumene hydroperoxide is produced. Cumene hydroperoxide then interacts with dilute acid to form phenols. Acetone is a prominent by-product of this process, demanding purification.

Synthesis Of Phenol:
1. Sodium benzenesulfonate pyrolysis:
Sodium hydroxide and benzene sulfonic acid are combined in this process. The solid sodium hydroxide is heated to a high temperature to fuse with the salt. After this reaction, you get sodium phenoxide acidified with water to obtain phenol.
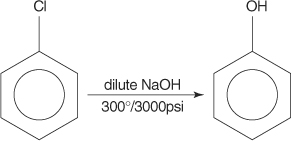
2. The Dow process:
Chlorobenzene is reacted with dilute sodium hydroxide at a temperature of 300°C and a pressure of 3000 psi in the Dow method. The Dow process is depicted below.
3. Air oxidation of cumene:
According to the cumene hydroperoxide production and degradation process, cumene (isopropyl benzene) oxidation produces both phenol and acetone in the presence of oxygen.
Hydroperoxide is produced in a chain reaction involving free radicals. When a free hydrogen radical is removed, it is replaced by another free radical known as a “tertiary” free radical. It’s time to start responding.
The free radical produced by the oxygen molecule is the next stage in the process. As a result of this attraction, the free hydroperoxide radical is generated.
![]()
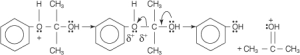
The second molecule of cumene, cumene hydroperoxide, and a third free radical are generated only when the hydroperoxide-free radical steals anhydrous free radical from the oxonium ion.
4. Cumene hydroperoxide degradation:
Cumene hydroperoxide is degraded through a carbocation process. Oxonium ions are generated in the first step. This occurs when the “hydroxyl group” of the hydroperoxide is attracted to a proton in the H3O+ molecule.
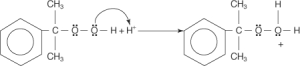
When water is removed from the equation, an oxonium ion is formed in its place.
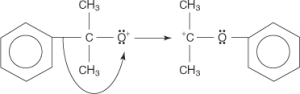
Phenide ions go to the oxygen atom, creating a tertiary carbocation. A new link to the ring can be formed between the (phenoxide ion) and a free electron bonding pair (phenyl group).
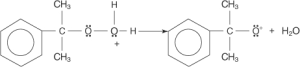
An oxonium ion is generated when an acid-base reaction with water stabilises the carbocation.
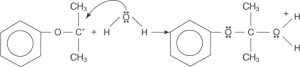
The oxonium ion is more stable after losing a proton.
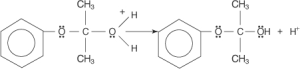
The oxygen-carbon bond’s electrons are attracted by the positively charged ether oxygen, which causes the charge to be delocalised across both atoms. The OH group’s non-bonding electron pair is drawn by a partial positive charge on the carbon atom to release the electrons from a previous oxygen-carbon connection.
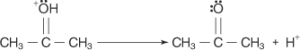
In the end, a proton is lost from the proton-rich acetone molecule, resulting in the production of acetone.
Uses Of Phenol:
➤More than 70 per cent of all phenol produced on the earth is used in the plastics sector to manufacture reagents.
➤Bisphenol A is commonly used in the polymer sector to manufacture different epoxide resins and polycarbonates by reacting phenol with acetone.
➤Bakelite is an example of a phenolic resin created when alkylphenols, phenol, or diphenols are mixed with formaldehyde. Bakelite is commonly used in electrical switches and automobiles because of its strong heat resistance and resistance to electricity and other chemicals. As a binding agent or adhesive, it is employed in various industries to remove electrons from the previous oxygen-carbon connection stabilisers.
➤It is employed in biomolecular research and extraction. Molecular biologists use phenol to extract nucleic acids from tissue samples for further study.
➤Precursors to several medications, including aspirin and many herbicides and pharmaceuticals, can be made from phenols.
Conclusion
Phenols are colourless, acidic, and needle-like solids. People used to make phenol from coal tar back in the day. It was a long and complicated process. However, new methods for synthesising phenols in laboratories have emerged with technological improvements
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out