The material ingredient that makes up the observable cosmos is known as matter. All objective phenomena are understood to be founded on matter and energy. The term matter is used in classical physics and general chemistry to refer to any material that has mass and occupies space by having volume. Matter is the “stuff” that makes up the universe because it takes up space and has mass. All matter is made up of atoms, which are made up of protons, neutrons, and electrons. Chemical energy is the force that holds atoms and molecules together. Everything that has mass and volume is defined as matter (takes up space). Most everyday goods that we deal with on a daily basis have mass and take up space, which is very easy to demonstrate.
Finally, all ordinary items that can be touched are made up of atoms, which are made up of interacting subatomic particles, and the term ‘matter’ is commonly used to refer to atoms and everything made up of them in both everyday and scientific contexts. Any particles (or a mixture of particles) that have both residual mass and volume. Photons and other energy phenomena or waves, such as light or sound, are not considered matter because they have no mass.
The structure of matter and the arrangement of particles change when the state of matter changes. The change in characteristics can be used to observe all of this.
States of Matter
One of the distinct forms that the many phases of matter adopt is the condition of the matter. In everyday life, four states of matter can be found: solid, liquid, gas, and plasma. Other states, such as quark–gluon plasmas, are predicted to be feasible but are currently only theoretical.
Solids have a tight atomic bond and a high viscosity, which results in a hard shape. The majority of solids are crystalline in the sense that they have a three-dimensional periodic atomic structure; nevertheless, some solids (such as glass) lack this periodic arrangement and are therefore non-crystalline or amorphous. The solid is densely packed with particles (ions, atoms, or molecules). The forces between the particles are so strong that the particles can only vibrate rather than move freely. As a result, the solid has a consistent, defined shape and volume. Solids can only be broken or sliced in order to change their shape.
A liquid is a nearly incompressible fluid that adapts to the shape of its container while maintaining an almost constant volume regardless of pressure. If temperature and pressure are constant, volume is defined. When a solid is heated past its melting point and the pressure exceeds the material’s triple point, it becomes liquid. Within a gas, the molecules have enough kinetic energy that intermolecular interactions have a negligible impact (or none at all in the ideal gas) and the typical distance between neighbouring molecules is substantially larger than the molecular size. The gas has no defined shape or volume, but it completely fills the container it is contained in.
Examples of Three States of Matter
There are three states of matter, and the descriptions of each are listed below:
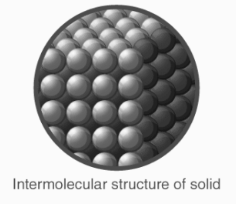
Solids
One of the fundamental states of matter is the solid state.
Solids are distinguished from liquids and gases by their rigidity.
Solids’ molecules are tightly packed and only oscillate around their mean positions due to strong intermolecular interaction.
Liquids and gases, on the other hand, have the quality of fluidity and may easily flow.
Solids are defined as states of matter with a rigid structure and a distinct shape and volume.
The least compressibility and thermal expansion are found in solids.
Consider iron (Fe)
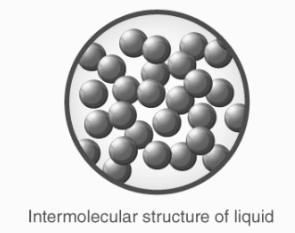
Liquids
Weak intermolecular interactions.
These forces are weaker than those of solids, but greater than gases.
Because liquids have a lot of room between their molecules, they flow more easily.
Solids are converted to liquids when their temperature is raised to the point where they begin to melt.
The density of liquids is usually somewhere between that of solids and gases. Liquids have slightly higher compressibility and thermal expansion than solids.
Consider water (H2O)
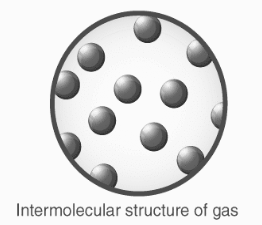
Gases
The distances between molecules are vast in this state of matter (intermolecular lengths are in the range of).
Translational, rotatory, and vibratory motions are hence predominant in gases.
Gases have no distinct shape or volume.
They have a high compressibility and thermal expansion as well.
Oxygen, for example (O2)
Conclusion
Solid, liquid, and gaseous are the three main states of matter. Everything we see in our daily lives is made out of matter, from ice cream to chairs to water. The intermolecular interactions and particle arrangement can classify matter into distinct states such as solid, liquid, and gas.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




