Nucleophilic Addition Reaction is a term used to describe the addition of nucleophiles to a nucleophile. As oxygen is highly electronegative, it forms a polar bond with carbon, in which the electrons are distributed unequally among the two molecules. As a result, the oxygen atom receives a partial negative charge, whereas the carbon atom receives a partial positive charge.
Since the carbon atom has a low electron density, it is vulnerable to attack by a nucleophile, which is an atom that can give electrons to the carbon atom. Also, aldehydes and ketones lack effective leaving groups when a nucleophile attacks the carbonyl carbon. Due to this, the nucleophile merely pulls the electrons toward the oxygen atom, forming a new bond between the carbon atom and the oxygen atom.
The oxygen atom now has a negative charge. It can attract a hydrogen ion from the solution, resulting in the formation of alcohol on the carbonyl atom. This type of reaction is referred to as a nucleophilic addition reaction, and it is particularly prevalent in the reactions of aldehydes and ketones.
When a nucleophile reacts with an electron-deficient species, a nucleophilic addition reaction occurs. A nucleophile creates a sigma bond with the electron-deficient species. They allow the conversion of carbonyl groups into various functional groups, which is useful in many applications.
![]() Aldehydes and ketones are examples of polar compounds. These compounds also have a greater boiling point than hydrocarbons, making them more suitable as fuels. Although aldehydes and ketones have lower boiling points than alcohols, they are more volatile. There are numerous reactions involving aldehydes and ketones that are adequate for various synthetic reactions to take place.
Aldehydes and ketones are examples of polar compounds. These compounds also have a greater boiling point than hydrocarbons, making them more suitable as fuels. Although aldehydes and ketones have lower boiling points than alcohols, they are more volatile. There are numerous reactions involving aldehydes and ketones that are adequate for various synthetic reactions to take place.
![]()
![]()
![]() Rate of Reaction:
The more electrophilic the substrate, faster the addition reaction. Also lesser the steric hindrance, faster the reaction. In accordance with both these factors, aldehydes are more reactive towards nucleophilic addition than ketones. Also resonance tampers with the electrophilicity of the substrate and reduces the rate of reaction.
Rate of Reaction:
The more electrophilic the substrate, faster the addition reaction. Also lesser the steric hindrance, faster the reaction. In accordance with both these factors, aldehydes are more reactive towards nucleophilic addition than ketones. Also resonance tampers with the electrophilicity of the substrate and reduces the rate of reaction.
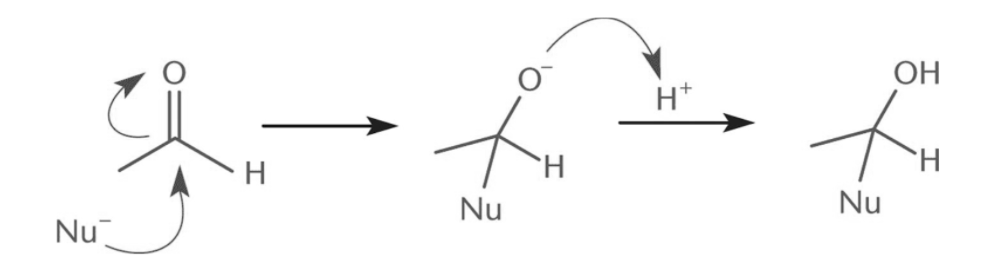
Mechanism of Nucleophilic Addition Reaction
In the case of nucleophilic addition, the mechanism is as follows: A two-step process is involved in the general mechanism of the nucleophilic addition reaction.- Step 1: Carbonyl is attacked by a nucleophile
- Step 2: There is no leaving group, thus addition occurs
 Aldehydes and ketones are examples of polar compounds. These compounds also have a greater boiling point than hydrocarbons, making them more suitable as fuels. Although aldehydes and ketones have lower boiling points than alcohols, they are more volatile. There are numerous reactions involving aldehydes and ketones that are adequate for various synthetic reactions to take place.
Aldehydes and ketones are examples of polar compounds. These compounds also have a greater boiling point than hydrocarbons, making them more suitable as fuels. Although aldehydes and ketones have lower boiling points than alcohols, they are more volatile. There are numerous reactions involving aldehydes and ketones that are adequate for various synthetic reactions to take place.
Nucleophilic Addition Reaction in presence of different agents
-
Nucleophilic Addition Reaction in presence of Water
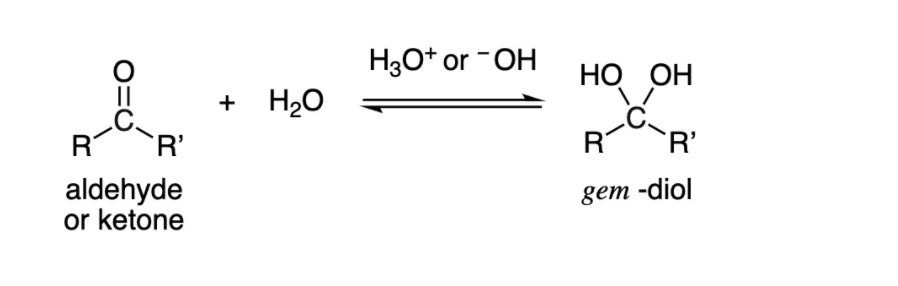
-
Nucleophilic Addition Reaction in the Presence of the Grignard Reaction

-
Nucleophilic Addition Reaction in presence of Primary Amines
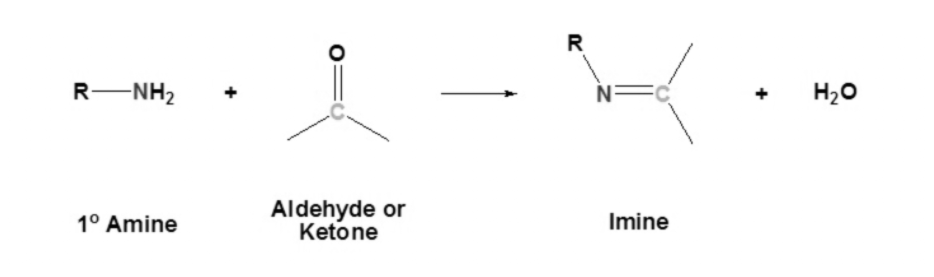 Rate of Reaction:
The more electrophilic the substrate, faster the addition reaction. Also lesser the steric hindrance, faster the reaction. In accordance with both these factors, aldehydes are more reactive towards nucleophilic addition than ketones. Also resonance tampers with the electrophilicity of the substrate and reduces the rate of reaction.
Rate of Reaction:
The more electrophilic the substrate, faster the addition reaction. Also lesser the steric hindrance, faster the reaction. In accordance with both these factors, aldehydes are more reactive towards nucleophilic addition than ketones. Also resonance tampers with the electrophilicity of the substrate and reduces the rate of reaction.
Points to Remember
- A nucleophilic addition reaction occurs when a chemical molecule with a double bond or triple bond combines with a nucleophile, resulting in the breakage of the double or triple bond.
- A carbocation will form in acetone, and the two CH₃ groups present in acetone will stabilise it, making the nucleophilic addition reaction a simple process.
- A carbonyl compound undergoes a nucleophilic addition reaction, and the steps involved in the mechanism are as follows: generation of the nucleophile, nucleophilic attack, protonation, and regeneration of a catalyst.
- Nucleophilic addition reaction occurs when the Carbonyl group is hybridised with Sp2 in the presence of Water. A breakdown of the pi link results in sp³ hybridisation of the carbonyl molecule.
- Comparatively, aldehydes are more reactive toward the Nucleophilic Addition Reaction than ketones.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




