In chemistry, an electrophilic addition is said to be done when an electrophilic species adds to a substrate containing a nucleophilic double or triple bond and results in the formation of two new sigma bonds. The basic steps involved in an electrophilic addition are:
CH2=CH2 + HCl → CH3-CH2Cl
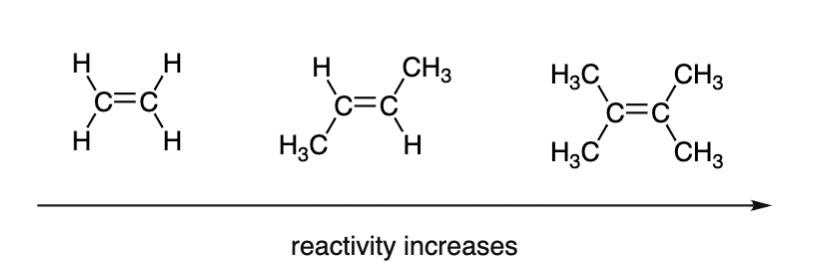
When treated with HX, alkenes from alkyl halides. Hydrogen halide reactivity towards the substrate is : HI > HBr > HCl > HF . The reactivity also increases if more alkyl groups are attached to the double bonded carbons. This is because the donating capability or nucleophilicity of the double bond increases.
![]()
Regioselectivity is expected by Markovnikov’s rule – ” the electron-rich component of the reagent adds to the carbon atom with fewer hydrogen atoms bonded to it,”. The reaction proceeds through protonation to give the more stable carbocation centre and is not stereoselective since the reaction proceeds through a planar carbocation.![]()
![]()
-C=C- + X2 → X-C-C-X
This proceeds through the cyclic halonium ion and thus a racemic mixture is formed as a product. Also 1,4 addition takes place in conjugated dienes. Usually CH2Cl2 is used as a medium for the reaction.![]()
![]() 1,4 Additions in conjugated systems
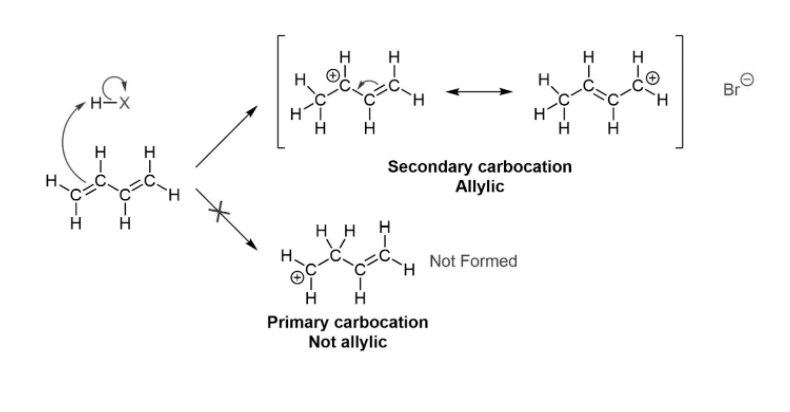
1,4 electrophilic addition occurs in conjugated double bond systems as it leads to greater stability in the transition state. The 1,4 addition occurs as a result of the resonance in the carbocationic transition state.
1,4 Additions in conjugated systems
1,4 electrophilic addition occurs in conjugated double bond systems as it leads to greater stability in the transition state. The 1,4 addition occurs as a result of the resonance in the carbocationic transition state.
![]() To see the difference between 1,4 addition and what we have been doing all along(1,2 addition), refer to the following diagram,
The selection between 1,2 and 1,4 also depends upon the temperature of the reaction mixture.
At low temperatures(ex. -78o C), we get a 1,2 addition product and at higher temperatures and room temperature, we get 1,4 addition as the major product.
The 1,2 product is also known as the kinetic product as it forms faster and 1,4 product is also known as the thermodynamic product as it is more thermodynamically stable.
The mechanism for 1,4 addition at room temperature is shown below:
To see the difference between 1,4 addition and what we have been doing all along(1,2 addition), refer to the following diagram,
The selection between 1,2 and 1,4 also depends upon the temperature of the reaction mixture.
At low temperatures(ex. -78o C), we get a 1,2 addition product and at higher temperatures and room temperature, we get 1,4 addition as the major product.
The 1,2 product is also known as the kinetic product as it forms faster and 1,4 product is also known as the thermodynamic product as it is more thermodynamically stable.
The mechanism for 1,4 addition at room temperature is shown below:
![]()
![]()
- Pi electrons from the unsaturated system attack the electrophile resulting in a carbocation or carbocation like species(ex. Halonium ion)
- A nucleophile attacks the carbocation.
Reaction of Alkenes with Hydrogen Halides
When alkenes are treated with hydrogen halides, it adds to give an alkyl halide as the product.CH2=CH2 + HCl → CH3-CH2Cl
When treated with HX, alkenes from alkyl halides. Hydrogen halide reactivity towards the substrate is : HI > HBr > HCl > HF . The reactivity also increases if more alkyl groups are attached to the double bonded carbons. This is because the donating capability or nucleophilicity of the double bond increases.
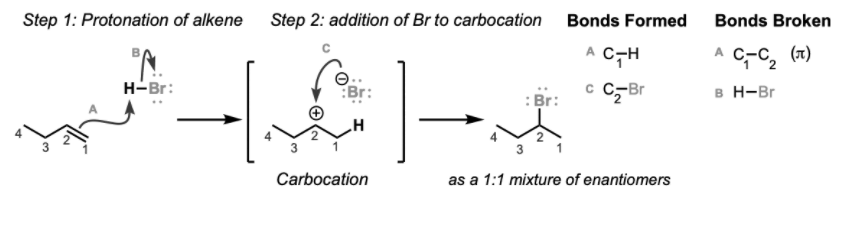
Regioselectivity is expected by Markovnikov’s rule – ” the electron-rich component of the reagent adds to the carbon atom with fewer hydrogen atoms bonded to it,”. The reaction proceeds through protonation to give the more stable carbocation centre and is not stereoselective since the reaction proceeds through a planar carbocation.

Framework for Reaction of Alkenes with HBr
Reaction type: Mechanism of electrophilic addition reaction- Stage 1: A destructive/base reaction. Protonation of the alkene to deliver a more consistent carbocation. The π electrons go about as a Lewis base.
- Stage 2: Attack of the nucleophilic bromide molecule on the electrophilic carbocation makes the alkyl bromide.
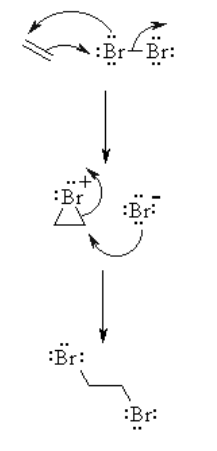
Halogenation of Alkenes
When a X2 molecule is added to a C=C, it forms two C-X sigma bonds.-C=C- + X2 → X-C-C-X
This proceeds through the cyclic halonium ion and thus a racemic mixture is formed as a product. Also 1,4 addition takes place in conjugated dienes. Usually CH2Cl2 is used as a medium for the reaction.
Framework for Reaction of Alkenes with Halogens
Reaction type: Mechanism of electrophilic addition reaction- Stage 1: The π electrons go probably as a nucleophile, attacking the bromine, dislodging a bromide molecule yet forming a cationic cyclic bromonium molecule as a midway.
- Stage 2: Attack of the nucleophilic bromide from the side away from the bromonium centre in an SN2 like plan opens the cyclic bromonium molecule to give by and large trans expansion.
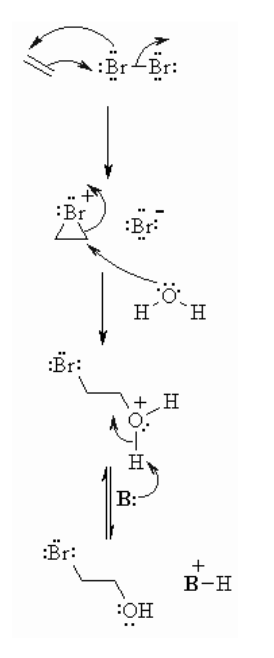
Halohydration of Alkenes
It involves the addition of X2/H2O to a double or triple bond system. This also proceeds with a halonium ion intermediate and involves anti addition to the unsaturated system.Component for Reaction of Alkenes with Br2/H2O
Reaction type: Mechanism of electrophilic addition reaction- Stage 1: Same initial step with respect to the response of Br2/CH2Cl2. The π electrons go about as a nucleophile, assaulting the bromine, uprooting a bromide particle yet framing a cationic cyclic bromonium particle as a half.
- Stage 2: Attack of the nucleophilic water particle from the side away from the bromonium focus in an SN2 like style opens the cyclic bromonium particle to give generally speaking trans addition.
- Stage 3: A corrosive/base response changes over the oxonium into liquor.
 1,4 Additions in conjugated systems
1,4 electrophilic addition occurs in conjugated double bond systems as it leads to greater stability in the transition state. The 1,4 addition occurs as a result of the resonance in the carbocationic transition state.
1,4 Additions in conjugated systems
1,4 electrophilic addition occurs in conjugated double bond systems as it leads to greater stability in the transition state. The 1,4 addition occurs as a result of the resonance in the carbocationic transition state.
 To see the difference between 1,4 addition and what we have been doing all along(1,2 addition), refer to the following diagram,
The selection between 1,2 and 1,4 also depends upon the temperature of the reaction mixture.
At low temperatures(ex. -78o C), we get a 1,2 addition product and at higher temperatures and room temperature, we get 1,4 addition as the major product.
The 1,2 product is also known as the kinetic product as it forms faster and 1,4 product is also known as the thermodynamic product as it is more thermodynamically stable.
The mechanism for 1,4 addition at room temperature is shown below:
To see the difference between 1,4 addition and what we have been doing all along(1,2 addition), refer to the following diagram,
The selection between 1,2 and 1,4 also depends upon the temperature of the reaction mixture.
At low temperatures(ex. -78o C), we get a 1,2 addition product and at higher temperatures and room temperature, we get 1,4 addition as the major product.
The 1,2 product is also known as the kinetic product as it forms faster and 1,4 product is also known as the thermodynamic product as it is more thermodynamically stable.
The mechanism for 1,4 addition at room temperature is shown below:

 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




