Lyophobic Multimolecular and Macromolecular Colloids
Introduction:
Colloids are heterogeneous systems where a particle (distributed phase) is dispersed into extremely small particles (dispersion medium). Because of their tiny size, colloidal particles contain a large surface area/unit mass.Their size of dispersed phase is larger than that in true solutions and smaller than those in suspension. Thus it has its own unique properties.
Classification based on the dispersion medium and dispersed phase in the physical state:
A colloidal system is a complex homogeneous fluid in which particles are evenly suspended throughout the fluid. Depending on the state of the dispersion medium and dispersed phase: solids, liquids or gases. There are eight different colloidal system types/states.
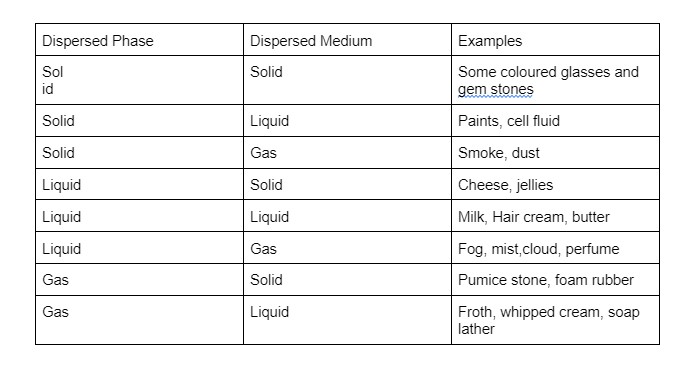
Classification depending on the nature of the relations between the dispersion medium and dispersed phase:
When it comes to colloidal state sols, there are two categories: those that are “lyophilic” (attracted to solvent) and those that are “lyophobic” ( those that are solvent repelling ).
Lyophilic Colloids:
Liquid love is what the word lyophilic signifies. Lyophilic sols are colloidal sols that are made by combining substances such as rubber, gum, starch, gelatin, and other similar compounds with an adequate liquid (in the form of the dispersion medium). The special feature of lyophilic colloids are that they are stable by itself due to stabilising interactions between the dispersed phase and dispersion medium
The sol can be retrieved by blending with the medium of dispersion again (for example, via evaporation). Also known as reversible sols, these sols may be used in both directions.
Lyophobic Colloids:
The term “lyophobic” refers to repelling liquids. Metals and some of their sulphide solids, for instance, will not form a colloidal suspension when simply dispersed in the medium used. Only a few procedures are used to make these type of colloidal suspensions. These suspensions rapidly coagulate when small quantities of electrolytes heat or shake them, and so they are unstable. Irreversible suspensions are another name for these suspensions. For the preservation of lyophobic suspensions, stabilising agents are required.
Macromolecular, Multimolecular, and related Colloids are classified depending on the kind of particles in the dispersed phase:
Colloids are characterised as multi-molecular, macromolecular colloids or related colloids according to the kind of particles in the dispersion phase.
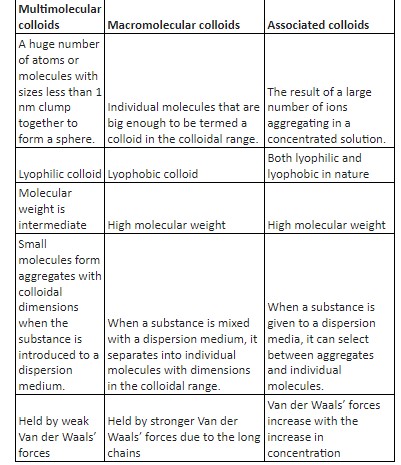
Define Multimolecular colloids:
High atom numbers or minor molecules of a material clump together during dissolution to generate a species with a colloidal size spectrum ( 1 – 1000 nm ).This is because individually the particles are too small to form a colloid. Multimolecular colloids are the result of this process. Gold sol, for example, might comprise a variety of sized particles with a large number of atoms.
Define Macromolecular Colloids:
Macromolecules in appropriate solvents generate colloids where the macromolecules’ sizes are in the colloidal range. Macromolecular colloids are a type of system like this. They are highly stable and, in many ways, reconstruct real solutions. Enzymes and proteins are examples of macromolecular compounds that exist naturally; nylon, polythene, polystyrene and artificial rubber are examples of macromolecular compounds that are synthesised. Also, macromolecular colloids are lyophobic.
Associated Colloids (micelles):
Associated colloids are those that are formed when the individual particles which are initially in solution state, combine to form a colloidal sized particle called micelle. However this happens only above a certain concentration and temperature. Examples of this are soap, detergent, etc.
Differences between multimolecular and macromolecular colloids:
Applications of Colloids:
Colloids are widely used in the industry.
Following are some examples:
- Electrical precipitation of smoke
- Purification of drinking water
- Medicines
- Tanning
- Cleansing action of soaps and detergents
- Photographic plates and films
- Rubber Industry
- Industrial products
Conclusion:
In this article, we learned about lyophobic multimolecular and macromolecular colloids. Multimolecular colloids are highly stable and, in many ways, reconstruct real solutions. Enzymes and proteins are examples of macromolecular compounds that exist naturally; nylon, polythene, polystyrene and artificial rubber are examples of macromolecular compounds that are synthesised.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




