Introduction
When a solution of anhydrous zinc chloride in concentrated hydrochloric acid is used to differentiate and categorise primary, secondary, and tertiary alcohols, it is referred to as the Lucas test.
This solution is referred to as the Lucas reagent in the scientific community.
A carbocation is formed as an intermediate in this reaction, and the reaction proceeds according to a unimolecular nucleophilic substitution reaction mechanism.
Because primary, secondary, and tertiary alcohols have varying degrees of reactivity with Lucas reagent, they produce a range of results when tested, which serves as the foundation for the Lucas Test.
It is possible to detect chloroalkane formation by looking for a change in colour of the sample from clear and colourless to turbid, which indicates the presence of chloroalkane formation.
Reaction with Lucas Reagent
A substitution reaction occurs in which the chloride group ultimately replaces the hydroxyl group.
Following the reaction, the solution changes from being clear and colourless to becoming cloudy, which indicates the formation of chloroalkanes.
Because the intermediate tertiary carbocation is much more stable in tertiary alcohol than in primary or secondary alcohol, the formation of tertiary alcohols from their respective halides occurs much more quickly than the formation of primary or secondary alcohols.
To make the reagent, equal amounts of concentrated HCl and zinc chloride are combined in a ratio of 1:1.
A reaction occurs when the chloride ion of hydrochloric acid reacts with an alkyl group of alcohol to form alkyl chloride, with zinc chloride acting as a catalyst in the reaction.
It is the difference in the rate of reaction of primary, secondary, and tertiary alcohols with Lucas reagent that serves as the foundation for the Lucas Test.
The straightforward reaction that takes place is depicted below.
RCl + H2O is formed by the reaction of ROH and HCl.
ZnCl2 is used in the presence of other metals.
Lucas Experiment
It is necessary to perform the Lucus test in order to distinguish between primary, secondary, and tertiary alcohols.
A difference in the reactivity of primary, secondary, and tertiary alcohols with hydrogen halide as determined by the SN1 reaction is used to determine the validity of this test.
In the presence of ZnCl2, the reaction ROH + HCl becomes RCl + H2O.
The difference in reactivity of the degrees of alcohol results in the different ease with which the corresponding carbocations can be formed.
Among the most stable tertiary carbocations are the primary carbocation, which is followed by the secondary carbocation, and the tertiary alcohols, which form the most stable tertiary carbocations.
The reagent used in the test is an equimolar mixture of zinc chloride and concentrated hydrochloric acid.
The alcohol becomes protonated, and the water molecule that is formed leaves, resulting in the formation of the carbocation.
The nucleophile Cl (which is present in excess) readily attacks the carbocation, resulting in the formation of the chloroalkane.
Because of the low solubility of the organic chloride in the aqueous mixture, the tertiary alcohol reacts immediately and completely within five minutes, as indicated by the presence of turbidity.
How to Carry Out the Lucas Test?
The Lucas test is carried out in the manner described below –
Preparation of Lucas Reagent –
Prepare a solution by mixing equal parts zinc chloride and concentrated HCl together.
In a test tube, place a very small amount of the sample that has been provided.
Now, pour approximately 2ml of the Lucas reagent into the test tube containing the given sample and thoroughly mix them together.
Keep track of how long it takes for the solution to become turbid or cloudy.
Equation
ZnCl2 + HCl is the formula for the Lucas reagent.
In this reagent, the chloride ion of hydrochloric acid reacts with an alkyl group or substitute alcoholic functional group to form an alkyl halide, with the catalyst zinc chloride acting as a cofactor.
Observations during Lucas test
The following are the results of the Lucas test if an unknown sample contains the following:
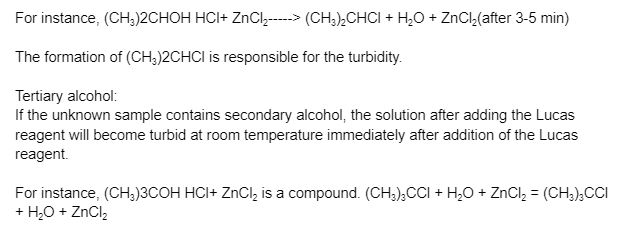
Primary alcohol:
if the unknown sample contains primary alcohol, the solution after adding the Lucas reagent will not become turbid at room temperature, indicating that the unknown sample contains primary alcohol.
However, if the solution is heated for a sufficient amount of time (30-45 minutes), turbidity in the solution will appear.
For instance, at room temperature, C2H5OH + HCl + ZnCl2 .
There is no turbidity.
Secondary alcohol:
The presence of secondary alcohol in an unknown sample will cause a turbid solution to form after the addition of Lucas reagent at room temperature after 3-5 minutes.
Conclusion
The Lucas test is used to differentiate between primary, secondary, and tertiary alcohols, as well as to determine which alcohol produces the fastest alkyl halide.
Using the Lucas test, you can determine the difference in reactivity between alcohols and hydrogen halide.
The rates at which primary, secondary, and tertiary alcohols react with hydrogen halide (hydrochloric acid) vary depending on the alcohol.
Anhydrous zinc chloride in concentrated hydrochloric acid is known as Lucas’ reagent, and it is used to prepare it.
This solution is used to classify alcohols with a low molecular weight, such as rubbing alcohol.
In this case, the chloride replaces a hydroxyl group, and the reaction is classified as a substitution.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




