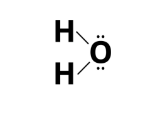
The Lewis electron dot structure, also known as a Lewis structure, is a representation of molecules that was developed in honour of the American chemist Gilbert Newton Lewis.
If the molecular formula of the compound is known, it is possible to draw electron dot structures or the Lewis dot formula for the compound.
Chemical bonding between atoms in a molecule is depicted by Lewis dot structures, also known as electron dot structures, which are diagrams.
Structures containing Lewis dot patterns are also referred to as electron dot patterns.
The total number of lone pairs present in each of the atoms that make up the molecule is also displayed on these graphs, as well.
Lewis dot structures, also known as electron dot structures or Lewis structures, are a type of crystal structure.
Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor, with the former being more common.
Lewis Dot Structure
It is the electronic structures of the elements, including how the electrons are paired, that are reflected in the Lewis dot structures.
It is possible to think of Lewis structures as “electron bookkeeping” because they are useful for summarising certain information about bonding.
Lewis dot structures are made up of dots that each represent an electron.
A bond is represented by a pair of dots between the chemical symbols for atoms.
Formula for the Formal Charge
It is possible to calculate the formal charge of an atom using the formula:
FC=V-{N+(B/2) }
The number of valence electrons of an atom in isolation is represented by V,
while the number of non-bonding valence electrons is represented by N,
and the total number of electrons in covalent bonds with other atoms in the molecule is represented by B in this formula.
The significance of the formal charge
In this case, the formal charge is a theoretical charge and does not reflect any real charge separation that exists in the molecule itself.
They aid in the selection of the lowest energy Lewis structure for a given molecule from among all of the possible Lewis structures.
Being familiar with the lowest energy structure of a reaction can aid in predicting the major product of a given reaction and can also aid in better understanding the phenomenon in question.
In general, the lowest energy structure has the smallest formal charge on the atom, as well as the most widely distributed charges throughout the structure.
Real Molecules vs. Lewis Structures:
Lewis structures are extremely useful when it comes to learning about oxidation states, valence, and the type of bonding that exists between two molecules.
Although there are many exceptions to the rule in reality, when it comes to the structure of reality, there are many.
Atoms, in general, attempt and seek to half-fill or completely fill the valence electron shell of their nucleus.
However, atoms are capable of doing so as well as forming molecules that are not stable.
Occasionally, the central atom can also produce more than the sum of the other atoms that are connected to it.
There is a possibility that the number of valence electrons will be greater than 8 electrons in total.
This is most noticeable in the higher atomic numbers of the elements.
Lewis structures are generally beneficial when dealing with lighter elements, but they are not particularly beneficial when dealing with transition metals, which include both actinides and lanthanides.
Their representation depicts the asymmetrical arrangement of electrons around the atom.
Conclusion
Lewis structure is a graphical representation of the electron distribution around an atom, and it is used in chemistry.
The most important reason for learning Lewis dot structure is that it aids in the prediction of the number and type of bonds that can be formed around an atom, which is extremely useful.
It also aids in the prediction of the molecule’s geometrical structure.
Lewis structures depict each atom and its position in the molecule’s structure by using the chemical symbol associated with that atom.
Lines are drawn between atoms that are chemically or physically bonded to one another (pairs of dots can be used instead of lines).
Lone pairs are formed by excess electrons in a molecule, and these lone pairs are represented as pairs of dots next to the atoms.
Main group elements in the second period and beyond typically react by gaining or losing electrons until they reach a valence shell electron configuration consisting of an entire octet of (8) electrons, but hydrogen (H) can only form bonds in which two electrons are shared.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out