The most abundant element in the earth’s crust is oxygen. Kari Scheele, a Swedish chemist, created oxygen for the first time in 1772 by heating mercuric oxide (HgO) and naming it vital air. Lavoisier, on the other hand, researched its essential essence and gave it the name oxygen, combining two Greek words oxys = sour and genus = producer (i.e, acid producer). It’s mostly found in the form of silicates, aluminates, and metal and non-metal oxides. It is found in the atmosphere at a volume proportion of 21.04 percent.
Definition
Oxygen is a colourless, odourless, gaseous element with an atomic number of 8 that makes up roughly 21% of the atmosphere’s volume and is biologically significant for its function in numerous biochemical and physiological processes, particularly in aerobic organisms. One of the chemical elements present in nature is oxygen. The pure substance of one sort of atom is referred to as a chemical element. Currently, there are 94 natural elements and 24 synthetic elements. Together with carbon, hydrogen, and nitrogen, oxygen is one of the most frequent components in living things. It is also the universe’s third most plentiful element, behind hydrogen and helium.
Oxygen 21% In The Earth Atmosphere
Because of small creatures known as cyanobacteria or blue-green algae, just 21% of the Earth’s atmosphere is made up of oxygen. Photosynthesis is a process in which bacteria use sunlight, water, and carbon dioxide to make carbohydrates and, yes, oxygen. In the atmosphere, oxygen makes up 21%, nitrogen makes up 78%, and other gases make up 1%. This is because oxygen is extremely reactive, whereas nitrogen does not react with anyone at room temperature. If the percentages of gases change in the opposite direction for oxygen and nitrogen, for example, if oxygen is 78 percent and nitrogen is 21 percent, there is a risk of fire on the earth’s surface since oxygen is reactive in the atmosphere and the amount of it is quite high. In this case, 78 percent N2 acts as an inert gas for atmospheric reactions, slowing down the reaction rate. As a result, oxygen levels are at a record high of 21%.
Geological Changes on Earth
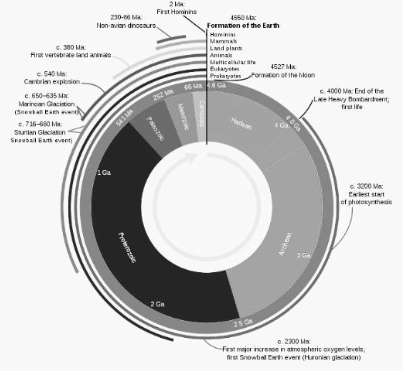
Based on the geological time scale, a system of chronological measurement based on the study of the planet’s rock layers, the geological history of Earth chronicles the key geological events in Earth’s past (stratigraphy). The solar nebula, a disk-shaped collection of dust and gas left over after the birth of the Sun, which also created the rest of the Solar System, accreted into Earth some 4.54 billion years ago.
Even though small cyanobacteria produced free oxygen as a byproduct of photosynthesis, Earth’s early atmosphere and oceans were devoid of the gas. Aerobic organisms require free oxygen, which is oxygen that has not been mixed with other components such as carbon or nitrogen. Small pockets of free oxygen began to form in the oceans about three billion years ago, signalling a shift. Then, some 2.4 billion years ago, the amount of oxygen in the atmosphere grew by 10,000 times in just 200 million years. The Great Oxidation Event significantly altered chemical processes on the Earth’s surface during this time.
Effect on Life
The Great Oxygenation Event had the first significant impact on the evolution of life. Many species that did not rely on oxygen to life died as a result of the rapid buildup of oxygen in the atmosphere. Large-scale evolutionary occurrences such as the Avalon explosion, the Cambrian explosion, trends in animal body size, and other diversification and extinction events are frequently considered as probable contributors to atmospheric oxygen concentrations.
The limiting role of diffusion in these organisms’ metabolism has been related to the huge size of many arthropods during the Carboniferous epoch, when the oxygen concentration in the atmosphere reached 35 percent. However, as Haldane points out in his essay, it would only apply to insects. However, the biological foundation for this link is shaky, and other lines of evidence suggest that oxygen concentration does not limit the size of current insects. Ecological restrictions, such as the introduction of flying competitors such as pterosaurs, birds, and bats, can better explain the tiny size of post-Carboniferous dragonflies.
Photosynthesizing Organisms
All oxygenic photosynthetic organisms absorb electrons and protons from water and utilise them to reduce NADP+ and plastoquinone, which are then used as energy sources in metabolic pathways like the Calvin cycle (CO2 fixation) and others.
Some organisms are capable of collecting sunlight’s energy and converting it to organic molecules. Photosynthesis is a vital part of life since it gives energy to both producers and consumers. Photosynthetic creatures, also called photoautotrophs, are photosynthesis-capable organisms. Higher plants, some protists (algae and euglena), and bacteria are among these creatures. Photoautotrophs, or photosynthetic organisms, collect sunlight’s energy and use it to make organic compounds through the process of photosynthesis. Photoautotrophs use the inorganic compounds of carbon dioxide, water, and sunshine to generate glucose, oxygen, and water during photosynthesis. Plants, algae, euglena, and bacteria are examples of photosynthetic creatures.
Examples Of Photosynthetic Organisms Include
Photosynthesis In Plants
In plants, photosynthesis takes place in chloroplasts, which are specialised organelles. Plant leaves contain chloroplasts, which contain the pigment chlorophyll. This green pigment absorbs light energy, which is required for photosynthesis. Chloroplasts have an internal membrane system made up of structures called thylakoids that function as light-to-chemical energy conversion sites. Carbon fixation, also known as the Calvin cycle, is the conversion of carbon dioxide to carbohydrates. Carbohydrates can be stored as starch, used as a source of energy during respiration, or used to make cellulose. The oxygen created during the process is released into the atmosphere through stomata, which are pores in the plant leaves. Plants play a significant role in the nutrient cycle, particularly in the carbon and oxygen cycles. By eliminating carbon dioxide from the air, aquatic plants and land plants (flowering plants, mosses, and ferns) contribute to regulating atmospheric carbon. Plants are also vital for the generation of oxygen, which is a beneficial by-product of photosynthesis and released into the air.
Photosynthesis In Euglena
Eukaryotic protists are Euglena. They are photoautotrophs with several chloroplasts in their cells. A crimson eyespot can be seen in each cell. Euglena are unicellular protists that belong to the Euglena genus. Because of their photosynthetic capacity, these creatures were placed in the phylum Euglenophyta with algae. Scientists now believe they are not algae, but rather a symbiotic association with green algae that gave them photosynthetic powers. Euglena has been assigned to the phylum Euglenozoa as a result.
Conclusion
The oxygen cycle refers to the movement of oxygen in various forms throughout nature. When it comes to uncombined elements in the atmosphere, oxygen is second only to nitrogen in terms of abundance. Plants and animals utilise oxygen to breathe and then release carbon dioxide into the air and water (CO2).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




