In the dehydration of alcohols procedure, which involves the use of E1 or E2 processes to cause water to be removed from the alcohol and the formation of a double bond, alkenes can be synthesized. The dehydration process of alcohols that results in the formation of alkene is carried out by heating the alcohols at high temperatures in the presence of a strong acid, such as sulfuric or phosphoric acid, to promote the reaction.
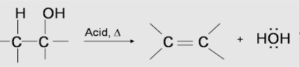
The required temperature range for the reaction reduces as the amount of hydroxy-containing carbon substituted increases. For example:
- 1° alcohol: 170° – 180°C
- 2° alcohols: 100°–140°C
- 3° alcohols: 25°– 80°C
It is possible that if the reaction is not sufficiently heated, the alcohols will not dehydrate to create alkenes but instead react with one another to form ethers (e.g., the Williamson Ether Synthesis).
Alcohols are amphoteric, which means that they may behave as both an acid and a base. Because of the lone pair of electrons on the oxygen atom, the –OH group is only weakly basic. Oxygen has the ability to transfer two electrons to a proton that is lacking in electrons. R—OH behaves as a base in the presence of a strong acid, protonating to form the very acidic alkyloxonium ion +OH2 when exposed to the acid (The pKa value of a tertiary protonated alcohol can go as low as -3.8). This fundamental property of alcohol is required for its dehydration reaction with an acid, which results in the formation of alkenes.
Mechanism for the Dehydration of Alcohol into Alkene
There are several different forms of alcohol, and each has a somewhat different method of dehydration. The main concept underpinning each dehydration reaction, on the other hand, is that the –OH group in the alcohol donates two electrons to H+ from the acid reagent, resulting in the formation of an alkyloxonium ion in the process. This ion performs admirably as a leaving group, resulting in the formation of a carbocation. Afterwards, the deprotonated acid (the base) combines with the hydrogen atoms adjacent to the carbocation, resulting in the formation of two double bonds.
Primary alcohols are eliminated by the bimolecular elimination (E2 mechanism), whereas secondary and tertiary alcohols are eliminated via the unimolecular elimination (E1 mechanism) (E1 mechanism). It is possible to rank the relative reactivity of alcohols in dehydration reactions in the following order:
Methanol < primary < secondary < tertiary
Primary alcohols dehydrate through the E2 mechanism
A proton from sulfuric acid (H2SO4) receives two electrons from the hydroxyl oxygen, resulting in the formation of an alkyloxonium ion. The conjugate base, HSO4–, then interacts with one of the neighboring (beta) hydrogen atoms, resulting in the formation of a double bond between the alkyloxonium ion and the adjacent hydrogen atom.
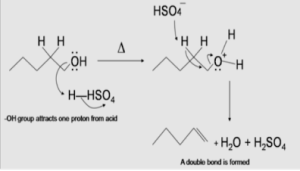
Secondary and tertiary alcohols dehydrate through the E1 mechanism
The process of secondary and tertiary –OH protonation results in the formation of alkyloxonium ions, which is similar to the reaction described above. But in this situation, the ion exits first and creates a carbocation as the reaction intermediate, rather than the other way around. The water molecule (which is a stronger base than the HSO4– ion) subsequently steals a proton from a neighboring carbon, resulting in the formation of a double bond between the two molecules. It is important to note that the alkene created is dependent on which proton is abstracted: the red arrows indicate the synthesis of the more substituted 2-butene, while the blue arrows indicate the formation of the less substituted 1-butene in the mechanism shown below. Remember that, according to Zaitsev’s Rule, the more substituted alkenes are generated preferentially because they are more stable than the less substituted alkenes, which is why they are more stable. To make matters worse, trans alkenes are more stable than cis alkenes, and they are also the most abundant product produced. In the example below, the trans diastereomer of the 2-butene product is the one with the greatest amount of abundance.
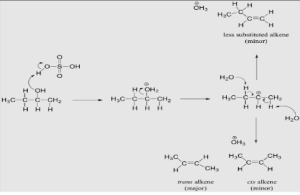
Dehydration reaction of secondary alcohol
Tertiary alcohol dehydrates in a manner similar to that of secondary alcohol.
A treatment with phosphorus oxychloride (POCl3) in pyridine can be used to achieve E2 elimination of 3o-alcohols in conditions that are not particularly acidic. This technique is also efficient with hampered 2o-alcohols; however, for unimpeded and 1o-alcohols, the substitution of the chlorophosphate intermediate with an SN2 chloride ion competes with the removal of the intermediate. Illustrations of these and related reactions are provided in the accompanying figure. The dehydration of a 3o-alcohol is depicted in the first equation. We believe that steric hindrance of the methylene group hydrogen atoms, which prevents the base from approaching the site of the non-Zaitsev product (less substituted double bond), is responsible for this predominance. The second example illustrates two different elimination processes that were used to the identical 2-alcohol mixture. The first employs the one-step POCl3 technique, which is particularly effective in this circumstance since SN2 substitution is delayed by steric hindrance An additional example of how an intermediate sulfonate ester might provide halogen-like reactivity to an alcohol is demonstrated by the second technique. The anionic leaving group is always the conjugate base of a strong acid, regardless of the situation.
CONCLUSION
Dehydration of alcohol is defined as a reaction in which alcohol combines with protic acid to lose water molecules and create alkenes as a result of the interaction between the two acids. Alternatively, dehydrogenation of alcohol is referred to as this process. A good illustration of an elimination reaction is this one. It demonstrates the distinction between primary, secondary, and tertiary alcohol. There are several different forms of alcohol, and each has a somewhat different method of dehydration. The main concept underpinning each dehydration reaction, on the other hand, is that the –OH group in the alcohol donates two electrons to H+ from the acid reagent, resulting in the formation of an alkyloxonium ion in the process.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




