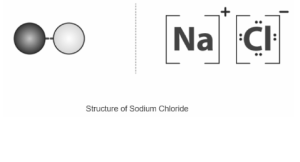
Sodium chloride, also known as salt, is an ionic compound with the chemical formula NaCl, which means there are equal amounts of sodium and chloride ions in it. Many multicellular organisms have extracellular fluid that is made up of sodium chloride. This salt is the main reason that seawater is salty. In the form of table salt, it is often used as a condiment and to keep food fresh. In many industrial processes, sodium chloride is used. It is a major source of sodium and chlorine compounds that are used as feedstocks for more chemical reactions.
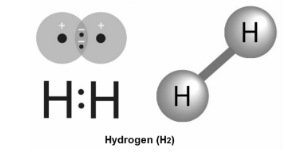
Hydrogen is a chemical element with the symbol H and the atomic number 1. When it comes to weight, hydrogen is by far the lightest. At normal pressure, hydrogen is a gas made up of molecules that have two atoms and have the formula H2. It is colourless,no smell, tasteless, non-toxic, and very flammable. Hydrogen is the most common chemical substance in the universe, making up about 75% of all normal matter. Stars like the Sun are mostly made of hydrogen in the plasma state.
Structure of Sodium Chloride and Hydrogen
Hydrogen:
The hydrogen atom has a nucleus that is composed of a proton that has one unit of positive electrical charge and an electron that has one unit of negative electrical charge. An electron is also connected with the nucleus that has one unit of positive electrical charge.
Sodium Chloride:
For example, sodium chloride is structured in a three-dimensional lattice structure, which is a regular repeating pattern of positive and negative ions that is a large three-dimensional lattice structure. The oppositely charged ions are held together in the enormous lattice by strong electrostatic forces of attraction, which are responsible for holding them together.
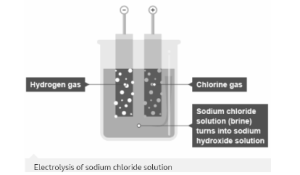
Reactivity of Sodium Chloride and Hydrogen
The production of hydrogen gas at the negative electrode and chlorine gas at the positive electrode occurs when an electric current is passed through a concentrated sodium chloride solution. The production of sodium hydroxide solution also occurs when an electric current is passed through a concentrated sodium chloride solution.
At the negative electrode, instead of sodium metal being deposited, hydrogen is released as sodium is too reactive.
During electrolysis, hydrogen ions H+(aq) (from the water) are expelled as hydrogen gas, H2(g) at the negative electrode
ions of chloride Cl–(aq) (from dissolved sodium chloride) is released as chlorine gas, Cl2(g), at the positive electrode
The sodium ions Na+(aq) (from dissolved sodium chloride) and the hydroxide ions OH–(aq) (from water) remain in the solution, forming sodium hydroxide, NaOH (aq).
At one of the electrodes in the electrolysis process, a half-equation depicts what’s going on. e– is the abbreviation used to represent electrons. Sodium chloride solution electrolysis half-equations are as follows:
When the H+ cations gain electrons, they are reduced at the cathode (negative electrode):
2H+(aq) + 2e– → H2(g)
When an ion loses electrons, it is oxidised at the anode (the positive electrode).
2Cl–(aq) → Cl2(g) + 2e–
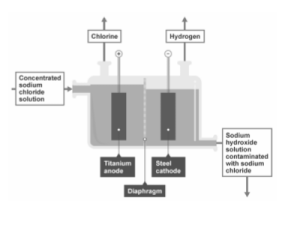
Industrial production of chlorine and alkali
On a large scale, industrial sodium chloride solution is electrolyzed using this procedure. A diaphragm cell is used to electrolyze concentrated sodium chloride solution, as indicated in the diagram.
CONCLUSION
Sodium Chloride has the chemical formula NaCl.Salt is another name for sodium chloride. It is found in seas and oceans. Additionally, it is found as rock salt. Between 1% and 5% of saltwater is composed of sodium chloride. It is a white crystalline substance. It is referred to as a saline solution when it is in its aqueous state. This water-soluble chemical is composed of sodium cation and chloride anion.
Salt and hydrogen gas are formed when sodium metal combines with hydrochloric acid. The salt formed is determined by which acid and which metal reacts.
This reaction produces hydrogen gas as well as sodium chloride when sodium metal is combined with hydrochloric acid. The reaction takes place in the presence of sodium metal and hydrochloric acid. The final products are hydrogen gas and a salt chloride solution.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out