In India, Egypt, Rome, and other ancient civilizations, borax or borax powder (chemical name sodium tetraborate decahydrate, chemical formulaNa2B4O7, 10H2O, or Na2B4O5OH4, 8H2O was widely used for the preparation of flux, glazes, and hard glass. The chemical element boron is represented by the inorganic compound borax.
Heating crystalline solid borax causes it to swell due to the loss of hydrated water, but heating it further produces meta-borate and boron trioxide. The chemical and physical properties of borax are extremely similar to those of boric acid. It’s made by boiling a solution of boric acid with sodium carbonate.
Chemical Reactivity of Borax with Oxygen
Borax swells when heated because it loses hydrating water, but additional heating produces the chemical compounds metaborate and boron trioxide. Borax fuses with metal oxides such as copper, iron, cobalt, nickel, or chromium oxide to generate a metal borate bead with a distinctive glossy colour. It’s used in chemistry to determine the chemical composition of metal salts. The borax bead test is the name of this test.
Na2B4O5OH4, 8H2O+fused →2NaBO2+ B2O3+ 2H2O
Na2B4O5OH4fused borax+CuO heating, oxidizing flame2NaBO2+CuBO22(green metaborate)+2H2O
Na2B4O5OH4 fused borax+CoP(heating, oxidizing flame) → 2NaBO2+ CuBO22(blue metaborate)+2H2O
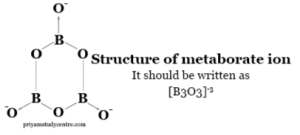
Formula Of Borax
Boron compounds have relatively similar chemistry, characteristics, and structure. The chemical characteristics of crystalline borax powder and boric acid are remarkably similar. These compounds are dissolved in a liquid solution, primarily hot water. The molecular formula of crystalline borax powder contains ten molecules of hydrated water at room temperature.
Na2B4O5OH4, 8H2O
Borax and Oxygen
Borax: Borax is a powdery white chemical that is also referred to as sodium borate, sodium tetraborate, or disodium tetraborate. It’s commonly used as a household cleanser and a laundry detergent booster. Boron, sodium, and oxygen are all found in this sample.
Borax is commonly discovered in dry lake beds, such as those found in California’s Death Valley, where water evaporated and left behind mineral deposits.
Boric acid is made from the same chemical compound as borax and has a similar appearance to it. Borax is often used for cleaning, although boric acid is mostly utilised as a pesticide. Boric acid attacks insects’ stomachs and neurological systems, killing them.
If ingested, both borax and boric acid in loose powder form can be dangerous, especially to children. They can irritate your skin as well.
Oxygen: O is a gaseous chemical element with the symbol O, an atomic number of 8, and a mass of 15,9994. It’s fascinating because it’s a necessary component of most live cells’ respiratory functions as well as combustion processes. It is the element with the greatest abundance in the Earth’s crust. Nearly a fifth of air is made up of oxygen. Non-combined gaseous oxygen is usually found in the form of diatomic molecules called O2, but it can also be found in the form of triatomic molecules O3 called ozone.
Oxygen is a colourless, odourless, and tasteless gas that condenses into a light blue liquid in its natural condition. Oxygen is the most paramagnetic among a small number of really paramagnetic gases. The paramagnetic property of liquid oxygen is minor.
Except for helium, neon, argon, and krypton, oxygen is a reactive gas that forms oxides with all other elements. At 20 degrees Celsius, it is fairly soluble in water (30 cm3 per 1 litre of water dissolves).
Conclusion
Borax is a hydrate salt of boric acid that is also known as sodium borate, sodium borate decahydrate, or sodium tetraborate decahydrate. It dissolves in water to generate a basic, aqueous solution and is commonly available in powder or granular form. It is soluble and has a wide range of industrial and home uses as a component in a variety of goods. Pesticides, metal soldering, glaze and enamel manufacture, tanning of skins and hides, artificial ageing of wood, as a preservative against wood fungus, analytical chemistry as a buffering agent, and pharmaceutical aid as an alkalizer are just a few of the applications.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






