Organic chemistry is the branch of chemistry that studies hydrocarbons and their derivatives. Because organic compounds are derived from living natural sources, they are thought to be fundamentally different from inorganic compounds.Some fundamental organic chemistry principles and techniques cover important topics such as organic compound shapes, nomenclature, isomerism, fundamental organic reaction mechanisms, electron displacement effects, and electromeric effect.
Tetravalency of carbon:
Tetravalency is one of the primary reasons why carbon can form so many different compounds. Carbon forms hybrid orbitals during the formation of a compound, and these molecules have a definite shape as a result of these hybrid orbitals. Carbon also forms a (pi) bond in addition to the sigma bond. The hybrid orbitals are not involved in this type of bonding, but they are formed by p-orbitals. The parallel orientation of p-orbitals on adjacent carbon atoms occurs in a bond.
Structural representations of Organic Compounds:
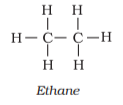
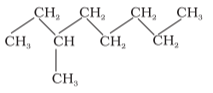
Structured formulas (complete, condensed, and Bond-line):
Carbon compound structures are represented in 2-dimensional geometry in three ways: complete structural formula, condensed structural formula, and bond-line structural formula.
Complete structural formula: As shown in the figure, different atoms are shown as being bonded to each other via a single, double, or triple bond in this structural representation.
Condensed structural formula: The bonds between the atoms are not shown in this type of representation, but the identical groups attached to an atom are represented by a subscript, as shown in the figure.
Bond-line structural formula: Carbon and hydrogen atoms are not shown in this type of structural representation; only dashes or bonds are shown. Other atoms or groups, such as chlorine, oxygen, and so on, are depicted.
Classification:
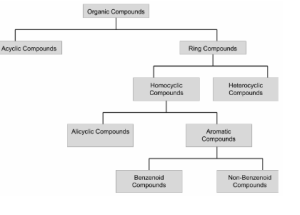
Isomerism:
Isomers are compounds with the same molecular formula but different physical and chemical properties, and the phenomenon is known as isomerism.
Isomerism is classified into two types. i) Constitutional isomerism; ii) stereoisomerism
Chain, nuclear, or skeletal isomerism: Isomers have the same molecular formula but differ in the nature of their carbon skeleton.
Position isomerism: Position isomerism occurs when different compounds from the same homologous series have the same molecular formula and carbon skeleton but differ in the position of a substituent, functional group, or unsaturated linkage.
Functional isomerism: It is said that functional isomerism exists when two compounds with the same molecular formula but different functional groups.
Metamerism: is a type of structural isomerism caused by an unequal distribution of carbon atoms on either side of the functional group or by different alkyl groups attached to either side of the same functional group and having the same molecular formula.
Tautomerism: A type of functional isomerism in which a single compound exists in two readily interconvertible structures that differ significantly in the relative position of at least one atomic nucleus, usually hydrogen. Tautomers are the two distinct structures.
Stereoisomers are isomers that have the same bond connectivity but a different arrangement of groups or atoms in space.
Geometrical isomers are stereoisomers with different group or atom arrangements around a rigid framework of double bonds. This type of isomerism occurs as a result of restricted rotation of double bonds or, in cyclic compounds, about single bonds.
Optical isomers are compounds that have the same physical and chemical properties but differ only in the rotation of the plane of polarized light. This phenomenon is known as optical isomerism.
Resonance:
The resonance structures (canonical structures or contributing structures) are fictitious and do not represent any real molecule on their own.The resonance stabilization energy, or simply the resonance energy, is the difference in energy between the actual structure and the lowest energy resonance structure.The resonance effect is defined as “the polarity produced in the molecule by the interaction of two -bonds or a -bond and a single pair of electrons on an adjacent atom.”The resonance or mesomeric effect is classified into two types: +R effect and -R effect.
Some basic principles and techniques:
There are some fundamental concepts that we must thoroughly understand.
Nucleophiles: A nucleophile is a reagent that is negatively charged or carries a single pair of electrons. These reagents draw protons or positive charges to themselves.
Electrophiles are positively charged reagents that require electrons to stabilize them.
The inductive effect occurs when two different atoms with different electronegativities are bonded together. The bonded electrons are shifted towards the more electronegative atom in this case, resulting in the polar covalent bond. When electrons shift towards carbon, this is known as the +I effect, and when electrons shift away from carbon, it is known as the -I effect.
–NH3+ > –NO2 > –SO2R > –CN > –SO3H > –CHO > –CO > –COOH > -F > –COCl > -CONH2 > –Cl > –Br > –I > –OR > -OH > -NR2 > –NH2 > –C6H5 > –CH=CH2 > –H
Electromeric Effect: In this effect, electrons are completely transferred to one of the atoms that are directly bonded with multiple bonds. This effect is only visible in multiple bonds. It is only active during the reagent attack and is only active for a short period of time.
Hyperconjugation: This effect results in the delocalization of sigma-electrons to the unsaturated system or unshared p-orbital. This effect is long-lasting.
Conclusion:
Organic chemistry is the study of the structure, properties, and reactions of organic compounds that contain carbon-carbon covalent bonds. Their structural formula is determined by structural analysis. Physical and chemical properties, as well as chemical reactivity, are studied to better understand their behaviour. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, as well as the laboratory and theoretical (in silico) study of individual organic molecules.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out