A crystal, also known as a crystalline solid, is a solid whose components (such as atoms, molecules, or ions) are organised in a highly ordered microscopic structure that forms a crystal lattice that extends in all directions. Furthermore, macroscopic single crystals are generally distinguished by their geometrical form, which consists of flat sides with distinct orientations. Crystallography is the scientific study of crystals and crystal formation. Crystallisation or solidification is the process of forming crystals through processes of crystal growth.
Unit cell
Crystal structure is a depiction of the orderly organisation of atoms, ions, or molecules in a crystalline substance used in crystallography. The inherent nature of the component particles results in symmetric patterns that recur along the primary directions of three-dimensional space in matter, forming ordered structures.
The unit cell of the structure is the smallest group of particles in the substance that makes up this repeating pattern. The symmetry and structure of the whole crystal are entirely reflected in the unit cell, which is built up by recurrent translation of the unit cell along its primary axes.
Crystal lattice
A crystal lattice, also known as a space lattice, is a pattern of points that repeats itself in space. We may pick a tiny section of the lattice to explain the space lattice well since it is a recurring configuration. When repeated in different directions, it generates the whole space lattice. The ‘unit cell’ refers to this little section of the lattice.
A crystal lattice or space lattice is a regular arrangement of the component particles — atoms, ions, or molecules – in a three-dimensional space.
Characteristics of Crystal lattice
A lattice point or lattice site is a point in the crystal lattice
A component particle, which might be an atom, a molecule (a collection of atoms), or an ion, is represented by each point in the crystal lattice
A crystal lattice is represented by a three-dimensional arrangement of lattice points
Straight lines connect the lattice points, highlighting the lattice’s geometry
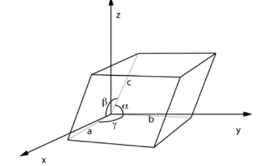
Lattice constants
One of the geometric parameters and angles that determines the geometry of the unit cells in a crystal lattice is the lattice constant or lattice parameter. The length a, b, and c of the three cell sides meeting at a vertex, as well as the angles α, β and γ between those edges, are the six lattice constants in three dimensions.
The length dimension exists in the crystal lattice constants a, b, and c. The metre is their SI unit, and they are typically measured in angstroms (A), which are 0.1 nanometres (nm) or 100 picometres (pm). The typical value is a few angstroms. In most cases, the angles α, β and γ are stated in degrees.
Crystal defects
A flaw in the regular geometrical arrangement of atoms in a crystalline material is called a crystal defect. Deformation of the solid, quick cooling from high temperatures, or high-energy radiation (X-rays or neutrons) impacting the solid cause these flaws. These imperfections in the solid can be found at single places, along lines, or over entire surfaces, and they affect its mechanical, electrical, and optical properties.
Types of defects
The Frenkel type, Schottky type, and impurity type are all examples of point defects. A single ion is displaced from its regular lattice location and shifts to a neighbouring interstice, or space, between atoms in the lattice, causing the Frenkel defect. Two ions of opposite charges escape the lattice in the Schottky defect. Foreign atoms that replace part of the atoms that make up the solid or squeeze into the interstices are known as impurity defects, and they have a role in the electrical properties of semiconductors, which are materials used in computer chips and other electronic devices.
Line defects, also known as dislocations, are lines in a solid along which entire rows of atoms are ordered abnormally. The ensuing spacing irregularity is most pronounced along a line known as the line of dislocation. Solids can be weakened or strengthened by line defects.
Surface flaws can occur where two grains, or tiny crystals, within a larger crystal, meet. A discrepancy across the grain boundary can occur when the rows of atoms in two separate grains run in slightly opposite orientations. Because the atoms on the surface modify their locations to compensate for the absence of nearby atoms outside the surface, the real exterior surface of a crystal is likewise a surface defect.
Conclusion
The crystal lattice is a three-dimensional symmetrical structural arrangement of atoms, ions, or molecules (component particles) as points inside a crystalline solid. It may be described as the geometrical organisation of the crystalline solids atoms, ions, or molecules as points in space.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out