Bohr’s Theory (also known as Bohr’s Atomic Model)
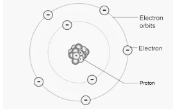
Both Thomson’s and Rutherford’s atomic models failed to provide satisfying answers to any questions about an atom’s energy or stability. Niels Bohr proposed an atomic structure model in 1913, describing an atom as a small, positively charged nucleus surrounded by electrons orbiting in circular orbits around the positively charged nucleus, similar to how planets orbit the sun in our solar system, with electrostatic forces providing attraction. Bohr’s atomic model is the name given to this model. It was essentially an improved version of Rutherford’s atomic model that overcame the original model’s restrictions. He agrees with him on the bulk of things, such as the concepts of a nucleus and electrons orbiting around it.
The Following Are Some of Niels Bohr’s Atomic Model’s Most Prominent Features
- Electrons spin in stable orbits around the nucleus, producing no radiant energy and remaining stationary in their orbits. Each orbit has a certain quantity of energy, known as an energy shell or energy level.
- The letters K, L, M, and N represent the number of shells in an orbit or energy level. When an electron is at its lowest energy level, it is considered to be in the ground state of the particle.
- When an electron moves from one orbit or energy level to another, it either emits or absorbs energy. Transitioning from a higher to a lower energy level causes energy to be emitted, whereas transitioning from a lower to a higher energy level causes energy to be absorbed (and vice versa).
The difference in the energies of the two energy levels (E1, E2), which are the same, equals the amount of energy received or emitted, according to Plank’s equation.
ΔE = E2-E1 = hv
Where,
ΔE = energy absorbed or emitted
Plank’s constant (h) and the frequency of electromagnetic radiation emitted or absorbed (v) are used to calculate E and H, respectively.
When an electron revolves in energy shells, its angular momentum is determined by the equation
mevr = nh/2π
Where n denotes the number of corresponding energy shells; 1, 2, 3,….. me denotes the mass of the electron, v denotes its velocity, r denotes its radius, and h denotes Plank’s constant.
The Atomic Model Theory of Bohr Has Some Limitations
- As a result, it is in violation of the Heisenberg Uncertainty Principle. According to the Bohr atomic model theory, electrons have both a known radius and orbit, i.e., a known position and momentum at the same time, which is contrary to Heisenberg’s hypothesis.
- In the case of smaller-sized atoms such as hydrogen, the Bohr atomic model theory made accurate predictions; but, when larger-sized atoms are taken into consideration, poor spectrum predictions are obtained.
- The Zeeman effect, which occurs when a spectral line is split into many components in the presence of a magnetic field, was unable to be explained by this theory.
- Moreover, it was unable to account for the Stark effect, which occurs when a spectral line is divided up into fine lines in the presence of an electric field.
Ernest Rutherford
Ernest Rutherford was a scientist who lived in the nineteenth century.
Rutherford’s early work resulted in the discovery of the concept of radioactive half-life, the radioactive element radon, and the differentiation and naming of alpha and beta radiation, among other things. The research for this project was carried out at McGill University in Montreal, Quebec, Canada. It served as the foundation for his Nobel Prize in Chemistry, which he received in 1908 “for his investigations into the disintegration of elements, and the chemistry of radioactive substances.” He was the first Oceanian to receive the award, and the first to carry out the work for which he was recognised in Canada. A member of the American Philosophical Society since 1904, he has made significant contributions to the field.
In 1907, he and Thomas Royds travelled to the United Kingdom, where they established that alpha radiation is made up of helium nuclei. At the time of their discovery, Rutherford and Royds were both members of the Royal Society. After winning the Nobel Prize in Physics, Rutherford finished his most famous achievement. Despite his inability to prove whether the charge was positive or negative, he theorised that atoms have their charge concentrated in a very small nucleus in 1911, and thus pioneered the Rutherford model of the atom through his discovery and interpretation of Rutherford scattering by Hans Geiger and Ernest Marsden’s gold foil experiment that same year. As proved by his studies, he created the first artificially induced nuclear reaction in 1917 when nitrogen nuclei were blasted with alpha particles. As a result, he found the emission of a subatomic particle he dubbed the “hydrogen atom” at the time, but eventually termed the proton once the proton was discovered in 1920.
Upon joining the Cavendish Laboratory at the University of Cambridge in 1919, Rutherford was appointed Director. During his tenure as director of the Institute, James Chadwick discovered the neutron in 1932, and in the same year, students working under his supervision, John Cockcroft and Ernest Walton, performed the first experiment to split the nucleus in a fully controlled manner. In 1937, he died and was buried in Westminster Abbey, next to Sir Isaac Newton, who was his mentor. In 1997, he was honoured by having the chemical element rutherfordium (element 104) named after him.
Antonius Johannes Van Den Broek
Antonius Johannes van den Broek (4 May 1870 – 25 October 1926) was a Dutch amateur physicist who discovered that the atomic number of an element corresponds to its charge. Henry Moseley found solid experimental proof for this idea in 1913, after it was published in 1911.
Scientific Breakthrough
It was published in Nature on July 20, 1911, barely one month after Rutherford revealed the findings of his experiments showing the existence of a tiny charged nucleus in an atom (see Rutherford model). However, Rutherford’s original study simply indicated that the nucleus’ charge was large, around half the atom’s atomic weight in whole number units of hydrogen mass. On this basis, Rutherford proposed that atomic nuclei are made up of helium nuclei, each with a charge equal to half their atomic weight. Smaller atoms’ nuclear charge would be approximately equivalent to their atomic number, with gold being an exception. For example, Rutherford found the gold charge to be around 100 units and thought it might be 98. (which would be close to half its atomic weight). But gold’s periodic table position (and consequently atomic number) was 79.
Thus Rutherford did not propose that the number of charges in an atom’s nucleus be equal to its position on the periodic table (atomic number). Van den Broek proposes this notion. Most physicists did not consider an element’s periodic table position (or atomic number) to be a physical feature at the time. Until Henry Moseley worked with the Bohr model of the atom, it was not known that atomic number was in fact a purely physical attribute (the charge of the nucleus) that could be measured. His study discovered (see Moseley’s law) the nuclear charge expressed by the Bohr equation as Z-1.
Conclusion
Both Thomson’s and Rutherford’s atomic models failed to provide satisfying answers to any questions about an atom’s energy or stability.
In the nineteenth century, Ernest Rutherford was a scientist.
Among other things, Rutherford’s early research led to the discovery of the idea of radioactive half-life, the radioactive element radon, and the distinguishing and naming of alpha and beta radiation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



