Red Phosphorus
Red phosphorus is one of the most prevalent allotropes of phosphorus and is regarded to be a derivation of the P4 molecule, which is a phosphorus atom with four protons. Amorphous (non-crystalline) networks of phosphorus atoms support the formation of phosphorus. According to the findings, it is more stable than white phosphorus (another naturally occurring phosphorus allotrope). The rich red colour and granular texture of red phosphorus distinguish it from other types of phosphorus.
The allotrope of red phosphorus is formed through the slow transition of white phosphorus. In the presence of light and energy in the form of heat, this transformation occurs more quickly. It is characteristically yellow in colour when a sample of white phosphorus has been largely transformed into red phosphorus via a reaction.
Structure
In the presence of red phosphorus, a polymeric chain of tetrahedrally structured P4 molecules forms, in which one of the P-P bonds has been broken, allowing for the connecting of these tetrahedrons. The chemical structure of red phosphorus is depicted in the diagram below.
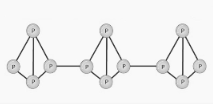
According to the image shown above, the structure of red phosphorus is pretty similar to the structure of a P4 molecule in terms of symmetry. Each phosphorus atom in P4 is bonded to three other phosphorus atoms in a tetrahedral form, which is the most stable structure known. In the event that one of these links is broken, these tetrahedral structures can proceed to form bonds with nearby P-atoms, resulting in the formation of a polymer-like structure in the process.
Properties of Red Phosphorus
Red phosphorus is colourless and odourless, and it has a rich red hue. In contrast to the white phosphorus allotrope, this allotrope is not toxic to human beings. It is crystallised when red phosphorus is heated to temperatures greater than 300 degrees Celsius. In its crystal lattice, it can also take on the shape of a cubic structure.It has a longer shelf life than white phosphorus.The molar mass of this compound is 30.974g/mol.It is classified as an amorphous solid.It has a melting point of 860 degrees Celsius.It has a density of 2.34 grammes per cubic centimetre.
This particular allotrope of phosphorus does not show any signs of phosphorescence (a type of photo luminescence). It should be noted that red phosphorus is less chemically reactive than its white phosphorus equivalent.
Production
When Anton von Schrotter, an Austrian chemist, heated white phosphorus to 300 degrees Celsius, he discovered red phosphorus for the first time. Other major methods of obtaining red phosphorus are explored in greater detail in this section..
From White Phosphorus
- The production of this red phosphorus begins with the heating of white phosphorus (which must be submerged in water) to 550 degrees Celsius in a steel kettle for 3-4 days, followed by cooling.
- Using a mechanism that condenses the reflux, it is possible to prevent the waste of phosphorus in the form of vapour.
- Following the 2-day mark, the temperature is raised to 673K in order to distil off any remaining white phosphorous that has remained.
- Following the removal of the water from the resulting combination, the addition of sodium carbonate and subsequent boiling of the mixture eliminates any lingering traces of the white allotrope that may have remained.
- In order to obtain the red phosphorus product, it is necessary to vacuum dry the mixture at this point.
From Bone Ash or Phosphorus-Rich Rocks
- It is necessary to produce a finely powdered powder from rocks that are high in phosphorus or from animal or fish bones in order to use this approach in the first phase.
- This powder/bone ash is treated with H2SO4 (sulphuric acid), which results in the formation of phosphoric acid and a small amount of calcium sulphate.
- Heat is applied to phosphoric acid in the presence of charcoal, resulting in the formation of white phosphorus, which can then be heated further to produce the needed red phosphorus product.
- When white phosphorus is exposed to sunlight, it undergoes a gradual transition that leads to the production of the red allotrope of phosphorus.
Applications
The red phosphorous and powdered glass that make up the striking surface of a matchbox are used to make it strike. Using this mixture, you can create a spark powerful enough to light a matchstick or lighter. The following are some of the other notable applications for red phosphorus.
- This allotrope of phosphorus is utilised in the ignition process of many flares, which are employed as emergency signals in many situations. Using this allotrope, it is also possible to maintain the flame’s combustion for an extended period of time.
- With the addition of magnesium and a binder, red phosphorus has the capability of being utilised as a smoke device to quickly create a smoke screen.
- Methamphetamine is also produced using this substance (commonly known as meth).
- Aside from its usage as a flame retardant in thermoplastics and thermosetting polymers, red phosphorus has other applications.
Conclusion
Red phosphorus is one of the most prevalent allotropes of phosphorus and is regarded to be a derivation of the P4 molecule, which is a phosphorus atom with four protons. Amorphous (non-crystalline) networks of phosphorus atoms support the formation of phosphorus. The allotrope of red phosphorus is formed through the slow transition of white phosphorus. In the presence of light and energy in the form of heat, this transformation occurs more quickly. It is characteristically yellow in colour when a sample of white phosphorus has been largely transformed into red phosphorus via a reaction. This allotrope of phosphorus is utilised in the ignition process of many flares, which are employed as emergency signals in many situations. Using this allotrope, it is also possible to maintain the flame’s combustion for an extended period of time. Aside from its usage as a flame retardant in thermoplastics and thermosetting polymers, red phosphorus has other applications.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




