Crystallisation
Crystallisation is a technique for purifying substances that is used in the pharmaceutical industry. A technique for separating solids from a solution is known as a separation technique.
It is possible to define crystallisation as the process by which atoms/molecules of a substance arrange themselves into a well-defined three-dimensional lattice and, as a result, reduce the overall energy of the system. When a substance is subjected to crystallisation, its atoms or molecules form strong bonds with one another at precisely defined angles to one another.
When a solid substance is added to a liquid and the mixture is stirred, the solid dissolves in the liquid. The problem is that when you add more and more solid to the liquid, there comes a point where no more solid can dissolve in the liquid. This point is referred to as the saturation point, and the fluid in which it occurs is referred to as the saturation solution.
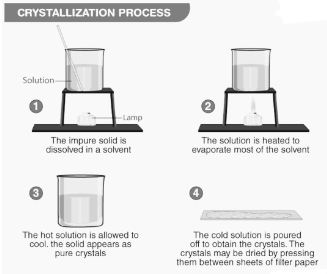
Crystallisation Process
- In an open container, the solution is heated to a point where the solvent molecules begin to evaporate, leaving the solutes behind.
- With cooling, solute crystals begin to accumulate on the surface of the solution, forming a layer of protection against the elements.
- Crystals are collected and dried according to the product specifications.
- The process of filtration separates the undissolved solids in a liquid from the dissolved solids.
- The size of crystals formed during this process is determined by the rate at which they are cooled.
- The rapid cooling of the solution results in the formation of numerous tiny crystals.
When cooling rates are slow, large crystals are formed.
The Crystallisation Activity Is Responsible for The Separation Of Substances
Here’s an experiment that will help you understand crystallisation better:
Step 1: Fill a beaker halfway with 50 mL of water.
Step 2: Pour in the sugar and stir it well.
Step 3: Now it’s time to heat the solution.
Step 4: Carry on with the process indefinitely.
Step 5: After a period of time, the amount of sugar that can be dissolved in water will reach a critical point. When the saturation point is reached, the solution is referred to as a saturated solution, and the solution is a saturated solution
Step 6: Using a filter paper, separate the sugar from the rest of the mixture.
Step 7: Pour the filtrate into a glass bowl and allow it to cool.
Step 8 :we will notice that some fine crystals have formed in the bowl.
Step 9: Filtration can be used to separate these crystals in step nine of the process. The liquid that remains after the crystals have been removed is referred to as mother liquor.
Illustrations of Crystallisation
It is referred to as crystallisation water because it contains a fixed number of water molecules in each unit of a salt’s formula. Another way of putting it is crystallised water that has been stoichiometrically bonded to form a crystal. Copper sulphate hydrate (CuSO4.5H2O) is an example of a chemical compound with this formula. Copper sulphate crystallises when it comes into contact with 5 molecules of water.
Salt crystallisation is the most practical application of crystallisation today, and it is also the most cost-effective method of producing salt currently available. The technology can also be used for compound purification and crystal synthesis, to name a few applications. It is also possible to define water of crystallisation as the water molecules that are responsible for the formation of a crystal’s structure. They are responsible for the formation and crystallisation of crystals. Copper sulphate (CuSO4. 5H2O) is a topical antibacterial and antifungal agent that can be applied to wounds and cuts.
Crystallisation Has a Variety of Applications
- The purification of seawater.
- Separation of alum crystals from impure samples using a filtration system
- Cryoprecipitation is used in the pharmaceutical industry as a separation and purification process for the synthesis and isolation of co-crystals, the purification of active pharmaceutical ingredients (API), the delivery of controlled release pulmonary drugs, and the separation of chiral isomers, among other things.
There you have it, a high-level overview of the crystallisation procedure.
Conclusion
Crystallisation is a technique for purifying substances that is used in the pharmaceutical industry. A technique for separating solids from a solution is known as a separation technique.When a solid substance is added to a liquid and the mixture is stirred, the solid dissolves in the liquid. The problem is that when you add more and more solid to the liquid, there comes a point where no more solid can dissolve in the liquid.It is referred to as crystallisation water because it contains a fixed number of water molecules in each unit of a salt’s formula. Another way of putting it is crystallised water that has been stoichiometrically bonded to form a crystal.Crystallization has a variety of applications: (i) The purification of seawater, (ii) Separation of alum crystals from impure samples using a filtration system.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




