Lanthanum is the first element in the third transition series, with one 5d electron and two 6s electrons. Cerium is the next element, and while it still has two 6s electrons, it has two electrons in the 4f orbitals and none in the 5d orbitals. There are seven distinct 4f orbitals, each of which can hold two electrons with opposing spins.
From cerium to lutetium, atoms have two to fourteen electrons in 4f- orbitals, respectively. These elements form the first inner transition series known as lanthanides, and despite the fact that lanthanum does not have any 4f electrons, it is customary to include it in this series.
Electron configuration of d block elements
D-block or transition elements are groups of three to twelve elements. In the periodic table, these elements are found between the p-block and s-block elements. The properties of these elements are intermediate between those of s-block and p-block elements. The properties of D-block elements change or transition from the most electropositive s-block elements to the less electropositive p-block elements. As a result, these elements are known as transition elements. Metals used in construction and manufacturing, metals valued for their beauty (gold, silver, and platinum), metals used in coins (nickel, copper), and metals used in modern technology are all included in the d-block elements (titanium).
The last differentiating electron is accommodated on penultimate d-orbitals in the transition element, and d-orbitals are successively filled. The following is the general electronic configuration of transition elements: (n-1), 1 to 10 and ns 1 or 2 There are four complete rows (called series) of ten elements each, each of which corresponds to the filling of 3d, 4d, 5d, and 6d-orbitals. Each series begins with a member of group three (IIIB) and concludes with a member of group twelve (IIB).
Electron configuration
Electron configuration describes the distribution of electrons within atoms and molecules among various orbitals (including shells and subshells).
There are four principle orbitals that are filled based on the element’s energy level and valence electrons. Each of the four orbitals can accommodate a different number of electrons. The s-orbital can hold two electrons, while the other three orbitals can hold six, ten, and fourteen electrons, respectively. The s-orbital denotes elements from groups 1 and 2, the p-orbital elements from groups 13, 14, 15, 16, 17, or 18, and the f-orbital elements from groups Lanthanides and Actinides. The main focus of this module, however, will be on the electron configuration of transition metals in the d-orbitals (d-block).
Transition metal electron configurations are unique in that they can be found in a variety of oxidation states. Although the elements can have a variety of oxidation states, they usually have a common oxidation state based on what makes that element the most stable. We will only work with the first row of transition metals in this module; however, the other rows of transition metals generally follow the same patterns as the first row.
On the periodic table below, the s, p, d, and f-orbitals are labelled:
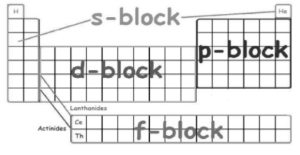
D block and f block elements
Based on this, they are classified as lanthanides or actinides. The 4f and 5f orbitals of f-block elements are consistently in the latter of two long periods. Based on this, they are classified as lanthanides or actinides. The transition elements’ d block elements have partially filled (n-1) d-orbitals. An element’s position in the periodic table is highly reflective of its properties and nature. Let’s take a closer look at the position in the periodic table.
Characteristics of f block
Elements with f-Block Characteristics
- They are extremely heavy metals. They are tough metals.
- They have high melting and boiling points in general.
- They have varying oxidation states.
- They produce coloured ions.
- They have a proclivity to form complex compounds.
- Actinides are naturally radioactive.
- Lanthanide oxides are used as abrasives in the cleaning of glass.
- They are magnetic in nature.
- As the atomic number increases, so does the atomic and ionic size.
- They contribute to the filling of (n-2) f orbitals.
- They have a high electropositivity and are highly reactive in nature.
Conclusion
Lanthanum is the first element in the third transition series, with one 5d electron and two 6s electrons. Cerium is the next element, and while it still has two 6s electrons, it has two electrons in the 4f orbitals and none in the 5d orbitals. Electron configuration of d block elements D-block or transition elements are groups of three to twelve elements. In the periodic table, these elements are found between the p-block and s-block elements. The properties of these elements are intermediate between those of s-block and p-block elements. The properties of D-block elements change or transition from the most electropositive s-block elements to the less electropositive p-block elements. The last differentiating electron is accommodated on penultimate d-orbitals in the transition element, and d-orbitals are successively filled.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



