Acids that can create more than one hydronium per molecule are known as polybasic acids.
By looking at various acids, such as sulphuric acid and phosphoric acid, we can see that each molecule contains more than one ionizable ion. Such acids are referred to as polybasic acids.
We utilise a variety of acids and bases on a daily basis, such as vinegar or acetic acid in the kitchen, boric acid in the laundry, baking soda in the kitchen, washing soda in the bathroom, and so on.Many acids and bases that we do not use in our daily lives are utilised in laboratories, including acids such as HCl, H2SO4, and bases such as NaOH, KOH, and others.
Some of these acids and bases simply need to shed one hydronium or hydroxyl ion, but the bulk of them need to shed several ions.We’ll study about acids and bases that have more than one ionizable ion per molecule in this section.
Ionization of acids:
When a chemical is exposed to a solution, it undergoes ionisation, which is the process by which neutral molecules are broken down into charged ions. Acids are chemicals that dissociate in an aqueous medium to form hydrogen ions, H+, according to the Arrhenius theory. Arrhenius’ hypothesis is useful in understanding acid and basic ionisation since most ionisation happens in an aqueous medium. The degree of ionisation of acids and bases can be used to determine the strength of acids and bases. Furthermore, between acidic and basic compounds, the degree of ionisation differs. A few acids, such as hydrochloric acid (HCl) and perchloric acid (HClO4), completely dissociate into their constituent ions in aqueous environments.
The acidity or baseness of an acid or base is measured by the degree of ionisation. In water, a strong acid completely ionises, whereas a weak acid just partially ionises. Because acids have different degrees of ionisation, they have different levels of weakness, which may be quantified. The chemical equation and an expression for the equilibrium constant for the ionisation of a weak acid are as follows:
H2O+HA(aq)H3O+(aq)+A–
[H3O+]=Ka[HA]/[A–]
The Equilibrium Constant for acid ionisation defines the Acid Ionisation Constant (Ka). The stronger the acid, however, the larger the acid ionisation constant. As a result, a strong acid is a better proton donor than a weak acid. The larger the acid ionisation constant, the stronger the acid, due to the concentration of the product in the numerator of the Ka (Ka).
Arrhenius explanation of acid and base ionisation
This reaction demonstrates that the acid dissociation equilibrium is dynamic, with proton transfer taking place both forward and backward. In comparison to H3O+, HA functions as a strong acid since it has a higher inclination to donate proton. Because the more powerful acid gives the more powerful base a proton. As the equilibrium alters, a weaker acid and weaker base are formed. The conjugate bases of strong acids are weaker than the conjugate bases of strong bases. This happens due to the high degree of ionisation of strong acids and bases.
Polybasic Acid Ionization
Consider the following ionisation process for a common polybasic acid.
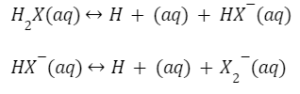
In this case, a dibasic acid is split into its constituent ions.
The equilibrium constant for the aforementioned reaction can be expressed as,
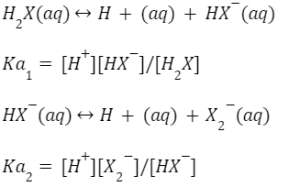
As a result, the product of the dissociation constants of the constituent ions multiplied together is the dissociation constant of polybasic acid.
Ka=Ka1×Ka2=[H+][HX–]/[H2X] × [H+][X2-]/[HX–] = [H+2][X2-]/[H2X]
Ka1 and Ka2 are the first and second ionisation constants of the acid H2X, respectively. We have three ionisation constants for a tribasic acid: Ka1, Ka2, Ka3, and so on. H2SO4, H2S, H3PO4 and other polybasic acids are examples.
H2SO4ionisation is as follows:
H2SO4 (aq)2H+(aq)+SO42-(aq)
Ka=[H+]2[SO42-]/[H2SO4)
Monobasic acids are formed when most inorganic acids react with bases in such a way that one atom of the acid is linked to one atom of a metallic oxide.
Some acids, such as pyrophosphoric, have one atom that has the ability to react with two atoms of the base, and these acids are referred to as dibasic acids. This class of acids includes tartaric and malic acids, which can be found in both the plant and animal kingdoms.
Conclusion :
Acids that can create more than one hydronium per molecule are known as polybasic acids. These are classified as dibasic, tribasic, and so on, depending on how many hydrogens they can replace. Polybasic acids include sulphuric acid and phosphorous acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



