Acids and bases have been defined numerous times and in a variety of ways. Numerous scientists have proposed various definitions for acids and bases, with some conceptions being highly specific and others being quite broad. Acids and bases are found in our daily lives. Except for water, every liquid we used had acidic or basic properties, such as vinegar (contains acetic acid), soft drinks (contains carbonic acid), buttermilk (contains lactic acid), and soap (contains lactic acid) (contains base). The initial definitions were based on their flavour and how they interacted with other substances.
Ionization of Acids and Bases
When a chemical is exposed to a solution, it undergoes ionisation, which is the process by which neutral molecules are broken up into charged ions. Acids, according to Arrhenius’s theory, are substances that dissociate in water to form hydrogen ions, H+. Due to the fact that the majority of ionisation happens in an aqueous medium, Arrhenius’s theory is critical for understanding acid and basic ionisation. Acids and bases can be classified according to their degree of ionisation. Additionally, the degree of ionisation changes according to the acidity or basicity of the substance. A few acids, such as hydrochloric acid (HCl) and perchloric acid (HClO4), completely dissociate into their constituent ions in aqueous environments.
Ionization of Acids
The degree of ionisation is a measure of an acid or base’s acidity or baseness. In water, a strong acid completely ionises, whereas a weak acid just partially ionises. Due to the fact that acids have variable degrees of ionisation, they also have varying degrees of weakness, which may be quantified. Due to the equilibrium nature of the ionisation of a weak acid, the chemical equation and expression for the equilibrium constant are as follows:
HA (aq) + H2O → H3O+ (aq) + A–
Ka = [H3O+] [A–] / [HA]
The Equilibrium Constant for Ionisation of an Acid is used to define the Acid Ionisation Constant (Ka). The greater the acid ionisation constant, however, the stronger the acid. As a result, a strong acid is a better proton donor than a weak acid. Due to the product’s concentration in the numerator of the Ka constant, the larger the acid ionisation constant, the stronger the acid (Ka).
Ionization of Bases
Certain bases, such as lithium hydroxide or sodium hydroxide, completely dissociate into their ions in aqueous solution and are referred to as strong bases. As a result, ionisation of these bases results in the formation of hydrochloric ions, represented by the symbol (OH–). An equivalent sentence for the bases is:
B + H2O ⇢ OH– + HB+
Kb = [OH–] [HB+] / [B]
The equilibrium constant for base ionisation is denoted by the abbreviation Kb. As a result, a strong base means that it is an excellent proton acceptor, whereas a strong acid implies that it is an excellent proton donor. In water, weak acids and weak bases dissociate as follows:
CH3COOH + H2O ⇋CH3COO‾ + H3O+
NH3 + H2O ⇋NH4+ (aq) + OH‾(aq)
The Arrhenius acid-base theory (the water-ion system)
Sweden Svante Arrhenius proposed the Arrhenius acid-base hypothesis. It was the first application of the acid-base idea in the modern era. This is a really straightforward and useful hypothesis. Acids, according to Arrhenius’s hypothesis, are substances that raise the concentration of H+ or proton in aqueous solution. The released H+ ion or proton is not a free-floating proton; it coexists with the water molecule and forms the hydronium ion (H3O+). Arrhenius acids include HCl (hydrochloric acid), H2SO4 (sulphuric acid), and HNO3 (nitric acid), among others.
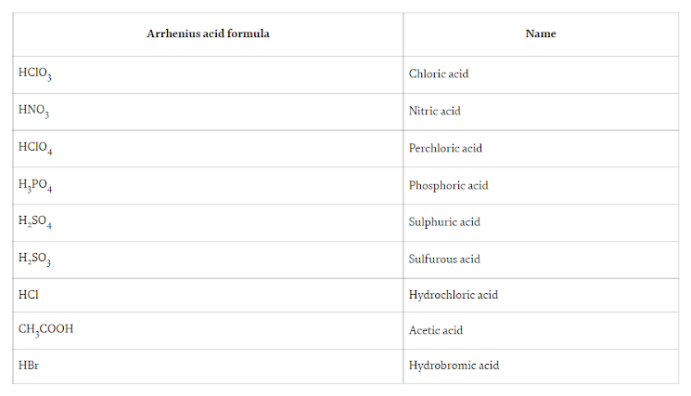
When it is Dissolved in water then:
HCl(aq) H3O+(aq) + Cl(aq)–
HNO3(aq) H3O+(aq)+ NO3–(aq)
Acids such as HNO3, HCl, and others dissociate into a single proton and are referred to as monoprotic acids. Acids such as H2SO4, H3PO4, and others that contain more than one hydrogen atom and produce more than one H+ ion upon dissociation are referred to as polyprotic acids. Polyprotic acids do not have to be stronger than monoprotic acids.
Similarly, Arrhenius bases are compounds that either enhance the concentration of OH or hydroxide ions in water or contain at least one OH ion in their formula. KOH (potassium hydroxide), NaOH (sodium hydroxide), Ca (OH)2 (calcium hydroxide), NH4OH (ammonium hydroxide) and Mg (OH)2 (magnesium hydroxide) are all examples of Arrhenius bases.
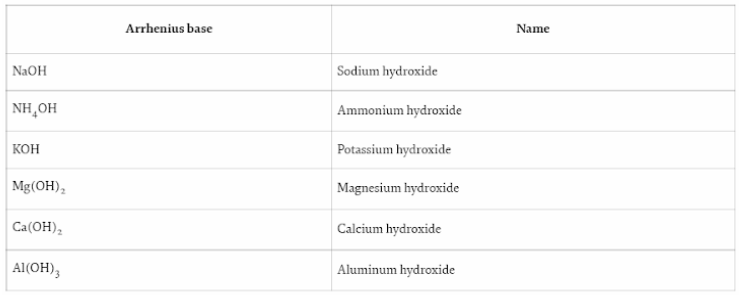
When sodium hydroxide is dissolved in water, it completely dissociates into the ions Na+ and OH–, increasing the concentration of hydroxide ions in solution.
Neutralization reaction
When Arrhenius acid and Arrhenius base react, salt and water are produced; this is referred to as the neutralisation reaction. For instance:
Strong acids are those that are totally ionised in water, such as HCl, HNO3, and H2SO4.
Hydrochloric acid is a highly corrosive acid. When it dissociates into water, it produces hydronium and chloride ions as byproducts. Although chloride ions are a weak base, their basicity does not make the solution basic because the acidity overcomes the chloride ions’ basicity. Hydronium ions are formed when H+ ions interact with water molecules. When a strong acid is used, the concentration of the hydronium ion generated is equal to the acid’s concentration, however when a weak acid is used, the concentration of hydronium ions in solution is always smaller than the concentration of hydrogen ions.
Whereas weak acids, such as acetic acid, are those that are weakly ionised in aqueous solution (CH3COOH).
The concentration of hydronium ions in weak acids is always less than the concentration of acid.
Similarly, bases that are entirely ionised in aqueous solution are referred to as strong bases, such as NaOH and KOH, whereas bases that are only partially ionised in aqueous solution are referred to as weak bases, such as ammonium hydroxide (NH4OH) and calcium hydroxide (Ca (OH)2).
Note: It is not required to concentrate strong acids/bases and dilute weak acids/bases. Because the dissociation of a drug is not concentration dependent.
Conclusion
The term “ionisation degree” is also used to refer to the fraction of neutral particles, such as those found in aqueous or gaseous solutions, that are ionised to form charged particles. For electrolytes, it might be defined as an acid or a base’s capacity to ionise itself. At times, a low degree of ionisation is referred to as partially or weakly ionised, and a high degree of ionisation is referred to as fully ionised. However, a fully ionised state can also signify that an ion has exhausted its electron count.
According to Arrhenius’ theory, an acid is a substance that dissociates in an aqueous medium to produce hydrogen ions. On the other hand, a base is a chemical that creates hydroxyl ions in an aqueous medium. Arrhenius’s hypothesis is particularly essential in understanding the ionisation of acids and bases. This is because ionisation frequently happens in watery media. The strength of an acid and a base can be assessed by the degree of ionisation of the acid and the base.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






