It is the temperature at which a concentrated solution may exist simultaneously in both its liquid and solid forms that is known as the melting point. A solid’s temperature will rise as more heat is added to it until it reaches its melting point. A greater amount of heat will then be required to transform the solid into a liquid without any change in temperature. Whenever the solid has completely melted, the temperature of the water will rise as a result of the extra heat. Pure compounds as well as elements can be distinguished by their melting temperatures, which are characteristic of crystalline solids. The majority of mixes and amorphous materials melt over a wide range of temperatures.It is generally assumed that the melting point of a solid is much like the freezing temperature of the correlating liquid; even so, since a liquid can freeze in a variety of crystalline forms and since contaminants decrease the freezing point, the real freezing temperature may vary from the melting temperature.
Solid to Liquid
Melting is the term used to describe the transformation of a solid into a liquid (an older term that you may see sometimes is fusion). Solidification is the procedure through which a liquid turns into a solid in the opposite direction. The temperature during which melting happens in just about any pure substance — known as the melting point — is perhaps a property of that substance. To transform a solid into a liquid, it is necessary to apply energy to the solid. A specific quantity of energy is required by every pure substance in order for it to change directly from a solid to a liquid. This quantity is referred to as the enthalpy of fusion (or heat of fusion) of the substance, and it is denoted by the symbol ∆Hfus. According to the paper “Enthalpies of Fusion for Numerous Substances,” it is thought that these numbers represent the substance’s melting point. It is important to note that the unit of ∆Hfus is kilojoules per mole, which means that we must understand the amount of materials in order to determine the amount of energy involved. The ∆Hfus is always represented as a positive number in the tables of the data. The process of solidification could be utilized in both the melting and the solidification processes, as long as you bear in mind that melting is always endothermic (i.e.,∆H will be positive), whereas solidification is indeed exothermic (i.e., ∆H will be negative).While melting, all of the energy is directed solely toward changing the state of the material; no energy is directed toward altering the temperature of the material. As a result, melting is an isothermal procedure since the temperature of the substance remains constant. Only until a material has completely melted could any extra energy go toward altering its heat.
Atmospheric Pressure
Pressure imposed by an atmospheric column on a unit area is referred to as atmospheric pressure, also termed barometric pressure, that is, the entire body of air above the specified area. With such a mercury barometer thus the term “barometric pressure,” that refers to the elevation of a mercury column which thus perfectly balances the weight of a column of atmosphere well over a barometer, it is possible to measure atmospheric pressure. Aneroid barometers, wherein the sensing device becomes an or even more empty, temporarily forced to evacuate, corrugated metal discs backed against breakdown by such an inside or outside spring, is also used to assess atmospheric pressure. This same transition in form of the disc to having to change pressure can indeed be noted using the pen arm as well as a clock-driven revolving drum.
Formula
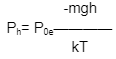
Here,
Ph– Ph is the pressure at the height h.
Po– Po is the pressure at sea level.
g- g is the gravitational acceleration.
k- k= Boltzmann’s constant (the ideal gas
constant divided by Avogadro’s
number), The constant of motion is
denoted by the letter k.
T- T denotes the absolute temperature.
m- m is the mass of a single air molecule.
Atmospheric pressure is measured in a variety of quantities, including millimetres (or inches) of mercury, pounds per square inch (psi), dynes per square centimetre, millibars (mb), standard atmospheres, and kilopascals. 760 mm (29.92 inches) of mercury, 14.70 pounds per square inch, 1,013.25 103 dynes per square centimetre, 1,013.25 millibars (one standard atmosphere), or 101.325 kilopascals is the normal sea-level pressure, according to the International Standards Organization’s definition. For instance, the difference between the highest and lowest recorded sea-level pressures is just 32.01 inches (in the heart of Siberia) and 25.90 inches (in the middle of the Pacific Ocean) (in a typhoon in the South Pacific). The little pressure changes that do exist on Earth play a significant role in determining the storm and high winds patterns that occur.
Equilibrium
Chemical equilibrium is a state of affairs that happens during the duration of a reversible chemical process where there is no net effect in the quantities of reactants and the products. The term “reversible” refers to a chemical reaction in which the products react with the original reactants as soon as they are created. Because both opposing reactions are occurring at the same rate, or at the same velocity, there really is no net effect in the quantity of chemicals involved when the two processes reach equilibrium. At this stage, the reactions can be deemed complete; that is, the maximum amount of reactants to outcomes has been converted under the conditions given in the process.
Conclusion
When two compounds melt at simultaneous temperatures, a combined melting point analysis can be used to determine if they are two different substances or one and the same material. The fusion heat of a mixture of the two elements is often lower than the fusion temperature of any of the individual components alone. A specific quantity of energy is required by every pure substance in order for it to change directly from a solid to a liquid. This quantity is referred to as the enthalpy of fusionMelting point depression is the term used to describe this phenomena.The melting point of the vast majority of pure materials is typically a single, strongly defined temperature value.The majority of mixes and amorphous materials melt over a wide range of temperature.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




